Introduction
The medical device engineering sector is rapidly evolving, driven by technological advancements and an increasing demand for innovative solutions to enhance patient care. Aspiring engineers in this field must navigate a complex landscape characterized by:
- Stringent regulatory standards
- Diverse career opportunities
- The necessity for continuous skill development
From understanding the intricacies of FDA regulations to mastering user-centered design processes, the path to becoming a successful product development engineer is multifaceted. This article delves into the essential qualifications, skills, and strategies required to thrive in this dynamic industry, highlighting the importance of:
- Hands-on experience
- Effective communication
- A commitment to lifelong learning
As the medical device market continues to expand, understanding these key components will be crucial for those looking to make a significant impact in healthcare technology.
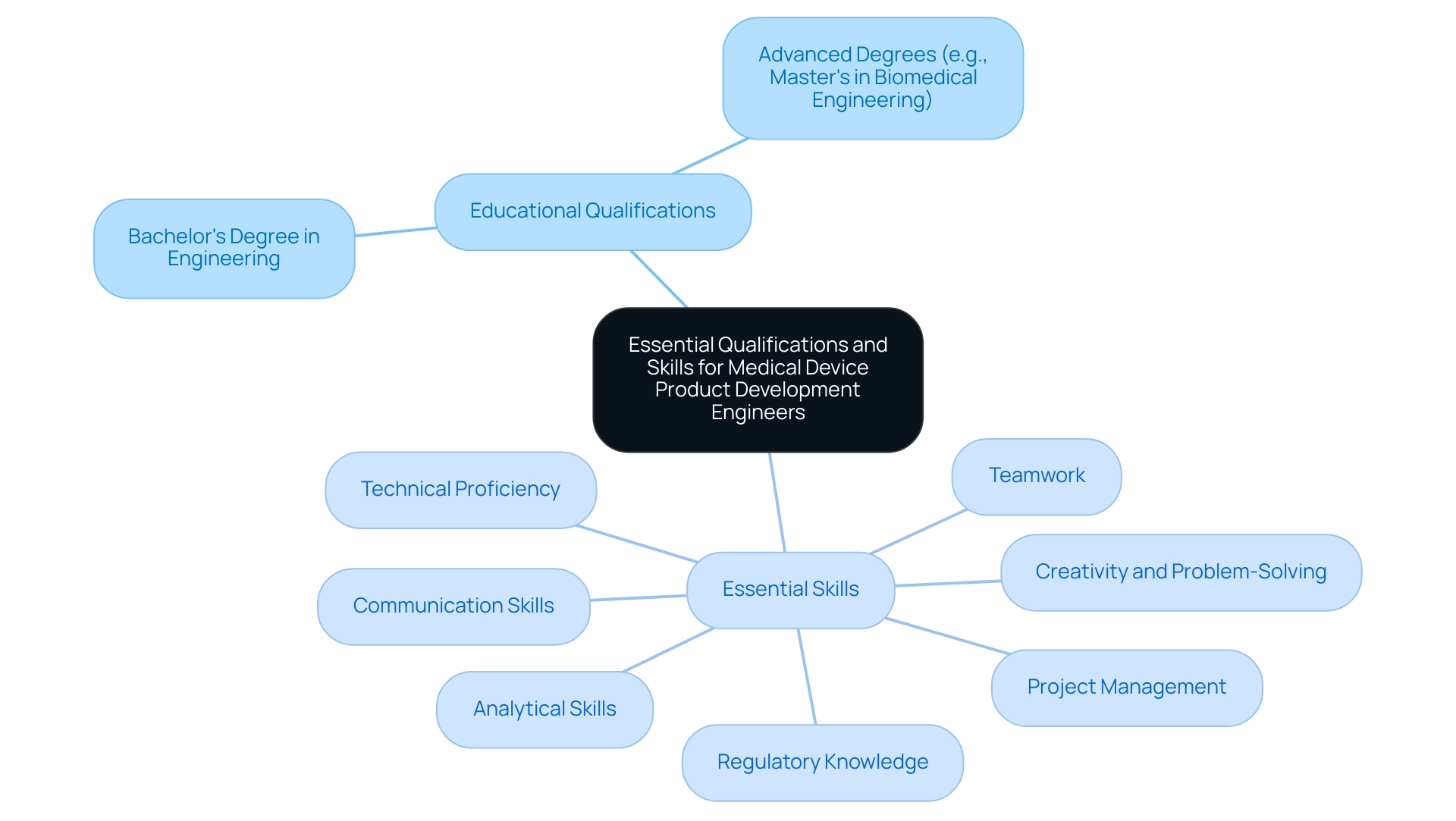
Essential Qualifications and Skills for Medical Device Product Development Engineers
To succeed as a product development engineer medical device in the healthcare equipment sector, individuals are typically expected to hold a bachelor's degree in engineering—whether mechanical, biomedical, or electrical—or a closely related field. Pursuing advanced degrees, such as a Master's in Biomedical Engineering, can significantly enhance career opportunities in this competitive field. The essential skills that candidates should cultivate include:
- Technical Proficiency: A solid grasp of CAD software, prototyping tools, and simulation software is vital for effective design and development.
- Analytical Skills: The ability of a product development engineer medical device to analyze complex data and tackle intricate engineering challenges is crucial for innovation in product development.
- Project Management: Experience in managing projects, including timelines and resource allocation, is necessary for a product development engineer medical device to ensure successful product launches.
- Communication Skills: Strong verbal and written communication skills are imperative for effective collaboration within diverse, cross-functional teams.
- Regulatory Knowledge: A comprehensive understanding of FDA regulations and industry standards governing health-related products is essential for product development engineers in medical device to navigate compliance successfully.
- Creativity and Problem-Solving: The ability to think creatively and solve problems is vital for a product development engineer medical device when developing innovative health-related products.
- Teamwork: Collaboration with cross-functional teams is essential for a product development engineer medical device in bringing a product from concept to market.
Furthermore, acquiring hands-on experience through internships or cooperative education programs can significantly enhance a candidate's qualifications, providing practical insights and skills that are highly valued in the industry. As we approach the Summer 2025 term starting on May 5, aspiring professionals should consider educational opportunities that will prepare them for success. Prepared to advance to the next stage in your healthcare equipment engineering career?
Zing Recruiting is here to help.
Navigating Your Career Path: Steps to Becoming a Product Development Engineer in Medical Devices
To successfully navigate your career as a product development engineer in the medical device sector, consider the following steps:
- Obtain Relevant Education: Begin with a bachelor’s degree in engineering or a closely related discipline. This fundamental knowledge is crucial for comprehending the complexities of healthcare equipment design and creation.
- Gain Experience: Actively pursue internships or entry-level positions in engineering or product development. These opportunities not only provide practical experience but also enhance your resume, making you a more attractive candidate for future roles as a product development engineer in the medical device sector. Recent statistics indicate that employment in numerous business professions, including biomedical engineering, is expected to expand more rapidly than average from 2023 to 2033, highlighting the importance of early career experiences. Significantly, the Bureau of Labor Statistics forecasts a 20% increase in demand for product development engineers in medical devices through 2022, emphasizing the robust opportunities in this sector.
- Develop Technical Skills: Participate in ongoing education to master pertinent software and tools utilized in the healthcare equipment sector. This ongoing education is crucial for a product development engineer medical device as technology evolves rapidly.
- Network with Professionals: Attend industry conferences and join professional organizations to establish valuable connections. Networking is pivotal for a product development engineer in the medical device field, as noted by Jeffrey S. Caudill, Corporate Safety and Health Managing Director, who emphasizes the importance of relationships in advancing one's career in engineering. Making connections can lead to mentorship opportunities, which are vital for career growth.
- Seek Certifications: Consider obtaining certifications in project management or healthcare regulations. Such credentials can significantly enhance your qualifications as a product development engineer medical device and demonstrate your commitment to professional growth.
- Apply for Positions: Actively look for job openings that align with your skills and interests. Customize your resume to emphasize pertinent experiences, particularly those acquired through internships, which frequently result in full-time roles as a product development engineer medical device in the field of health technology.
- Prepare for Interviews: Practice common interview questions and be prepared to discuss your technical skills and relevant projects comprehensively. This preparation can set you apart from other candidates.
- Continuously Improve: Stay informed about industry trends and advancements. As a product development engineer medical device, keeping abreast of new developments will ensure you remain competitive in this dynamic field. As indicated by the Bureau of Labor Statistics, the need for healthcare technology professionals is expected to increase, making it essential to maintain your skills relevant and current. Furthermore, examine the case study named 'Medical Equipment Design and Development,' where biomedical engineers utilize their knowledge to create products and software that address the requirements of patients and healthcare providers, demonstrating the practical application of the skills you gain.
Understanding Regulatory Standards in Medical Device Development
In the healthcare equipment sector, navigating regulatory standards is crucial for ensuring product safety and effectiveness. Engineers must be well-acquainted with the following core regulations:
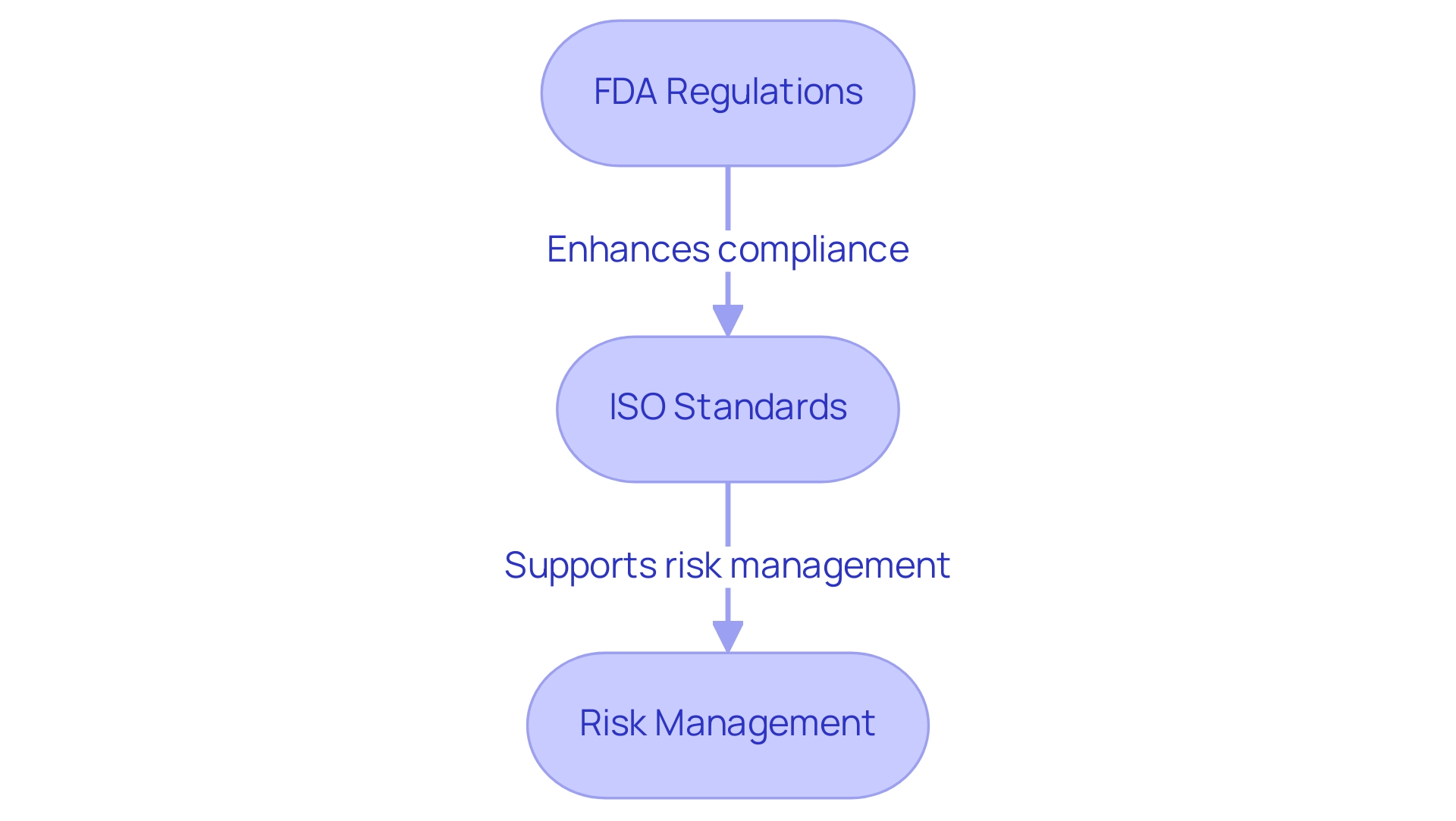
- FDA Regulations: The Food and Drug Administration (FDA) plays a critical role in the approval and oversight of medical devices. Engineers are required to adhere strictly to established guidelines for design, testing, and manufacturing. Notably, for the year ending September 2022, 67 percent of FDA 510(k) submissions faced requests for additional information during the substantive review process, highlighting the importance of thorough documentation and compliance. Any establishment where items are manufactured, processed, packed, installed, used, or implanted, or where records of results from the use of items are kept, can be subject to inspection, emphasizing the regulatory scrutiny that engineers must navigate. Ana Criado, with her extensive experience at INVIMA, emphasizes that understanding these regulations is crucial for successful product creation.
- ISO Standards: The International Organization for Standardization (ISO) establishes key standards that govern quality management systems in the healthcare equipment sector, particularly ISO 13485. Implementing these standards not only ensures quality but also enhances operational efficiency. Companies that have successfully integrated ISO 13485 into their processes have reported significant improvements in compliance and product reliability. Ana has consulted with various organizations to help them achieve ISO compliance, highlighting the practical implications of these standards in the Colombian market.
- Risk Management: Adhering to ISO 14971 is essential for effective risk management in healthcare product development. This standard assists engineers in recognizing and reducing potential risks related to healthcare equipment, promoting a culture of safety throughout the product lifecycle. Additionally, the Patient Problem Code data element, available in the ASR_PPCs zip file, serves as a valuable resource for analyzing and addressing patient-related issues. As demonstrated by the MAUDE Patient Data, which includes a significant number of records, comprehending patient issues is crucial for enhancing safety and effectiveness.
Recognizing the importance of regulatory frameworks, experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, emphasize the necessity of compliance within the Colombian context. "Navigating the complexities of regulatory standards is not only about compliance; it's about ensuring the safety and efficacy of health equipment that ultimately affects patient well-being," she states. INVIMA, the Colombia National Food and Drug Surveillance Institute, plays a pivotal role as a Level 4 health authority, overseeing the regulation and approval of healthcare products, thereby ensuring their safety and efficacy in the market.
This classification signifies INVIMA's competency in executing health regulation functions recommended by PAHO/WHO, which is crucial for maintaining high standards in oversight of health products. By remaining knowledgeable about these regulations, product development engineers in medical devices can greatly aid in the creation of secure and efficient healthcare tools while reducing the risk of expensive compliance failures. For instance, the FDA's 510(k) approval process, which is the most common regulatory pathway for low-to-moderate risk products, has an average decision time that can extend beyond the expected 90 days, reflecting the complexities involved in regulatory compliance.
The average time to receive a decision on 510(k) applications was 147 days in 2021, indicating that the actual time is closer to five months. Grasping these dynamics is essential for success in the healthcare equipment sector.
Innovative Design and Development Processes in Medical Devices
Innovative design and development processes are essential for the creation of effective healthcare products at bioaccess™. By joining our dedicated team, you can contribute to the revolution in healthcare technologies, ensuring that quality and impact are at the forefront of our efforts. Key components of these processes include:
- User-Centered Design: Actively involving end-users in the design process ensures that healthcare products are tailored to meet their specific needs, ultimately enhancing usability. A recent study on the UCD-11 measure retained 11 items from 19 identified variables, demonstrating a three-factor structure that explained 68% of the variance in user experience. The percentile ranks for UCD-11 scores indicate how these scores compare within the data set, providing valuable insights into the effectiveness of user-centered design. Furthermore, the study demonstrated acceptable internal consistency with a Cronbach alpha of .72, underscoring the reliability of the findings and the importance of incorporating user feedback into design.
- Prototyping and Testing: Rapid prototyping is a vital approach that allows engineers to create and evaluate models swiftly. This iterative process encourages continual enhancements based on user insights, leading to more refined products. Specialists in the area concur that effective prototyping greatly enhances success rates in healthcare technology engineering.
- Collaboration Across Disciplines: Involving professionals from different fields—such as engineering, healthcare, and design—fosters creativity and leads to better product outcomes. This interdisciplinary partnership is crucial for tackling the diverse challenges in healthcare equipment development.
- Systems Engineering: Applying systems engineering principles aids in managing the inherent complexity of healthcare projects, ensuring that all components operate cohesively. Such an approach is crucial in attaining the desired performance and reliability in healthcare instruments.
Additionally, the significance of the Statistical Heuristic Assessment technique in user experience consulting further highlights the importance of UCD in healthcare product creation, reflecting modern practices in the field.
By adopting these innovative methods, the product development engineer medical device team at bioaccess™ can substantially improve the quality and effectiveness of healthcare tools. As highlighted by Creanova, 'At Creanova, we believe it is crucial to apply UCD during the design and development phase of health-related products as this guarantees efficient, effective, but above all satisfying solutions, keeping users pleased in the long term.' Join us as a product development engineer medical device to help shape the future of healthcare through your expertise.
APPLY TODAY! At bioaccess™, we distinguish ourselves as a vetted CRO and consulting partner for U.S. healthcare companies in Colombia, offering unmatched support and expertise to advance your projects.
Exploring Career Opportunities and Market Trends in Medical Device Engineering
The area of healthcare equipment engineering is experiencing substantial change, offering numerous career prospects for experts, especially in Colombia, where INVIMA has an essential function. INVIMA (Colombia National Food and Drug Surveillance Institute) supervises the regulation of health products, ensuring adherence to health standards and best practices. This regulatory framework not only safeguards public health but also fosters an environment conducive to job creation and economic growth within the Medtech sector.
Key trends influencing the market include:
- Increased Demand: The combination of an aging population and rapid technological advancements is driving the need for innovative medical devices. According to the 18th annual Pulse of the MedTech Industry report, breakthroughs in MedTech AI are expected to create numerous job opportunities, reflecting the growing number of openings for engineers equipped with the skills to meet these challenges.
- Diverse Roles: There is a broad spectrum of opportunities available across various specializations, including design engineering, quality assurance, Regulatory Affairs, and project management. Each role is essential in the creation and implementation of healthcare tools, contributing to the overall success of the sector. The regulatory expertise provided by INVIMA, particularly through its Directorate for Medical Equipment and other Technologies, is essential in navigating these roles effectively by monitoring and controlling medical products and suggesting technical standards for their manufacturing and marketing.
- Emerging Technologies: Innovations in telemedicine, wearable devices, and personalized medicine are creating new pathways for product development professionals. These fields not only improve patient care but also increase the technical requirements for professionals, making adaptability essential. As highlighted in the Pulse report, companies must adopt digital transformation, artificial intelligence, and automation to remain competitive in the medtech industry.
- Global Market Expansion: As companies increasingly seek to penetrate international markets, there is a heightened demand for professionals who possess a thorough understanding of global regulatory frameworks and practices. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores the importance of regulatory knowledge for ensuring compliance and facilitating smooth market entry. The increasing popularity of at-home diagnostics, especially during the pandemic, further emphasizes the necessity for professionals who can navigate these evolving landscapes.
As stated by EY, "the insights and services we provide help to create long-term value for clients, people and society, and to build trust in the capital markets." To effectively navigate the evolving job landscape, aspiring product development engineer medical device professionals must remain informed about these trends and the regulatory landscape shaped by INVIMA, positioning themselves strategically to capitalize on emerging opportunities in the field. Additionally, leveraging platforms like AlphaSense can provide companies with a competitive edge through smarter decision-making and enhanced research capabilities.
Conclusion
The medical device engineering sector presents a dynamic and rapidly evolving landscape that requires aspiring engineers to be well-prepared for the challenges ahead. The journey begins with obtaining the essential educational qualifications, typically a degree in engineering, and continues through the development of key skills such as:
- Technical proficiency
- Analytical thinking
- Effective communication
Hands-on experience through internships is invaluable, providing practical insights that enhance employability in this competitive field.
Navigating the intricate regulatory standards is another critical component of success in medical device development. Familiarity with FDA regulations and ISO standards ensures that engineers can design safe and effective products while maintaining compliance with industry requirements. As highlighted, a comprehensive understanding of these regulations not only facilitates product approval but also safeguards patient health, underscoring the importance of regulatory knowledge in this sector.
Innovation is at the heart of medical device engineering, driven by user-centered design processes, rapid prototyping, and interdisciplinary collaboration. Embracing these practices fosters creativity and enhances the quality of medical devices, ultimately leading to improved patient outcomes. As the demand for medical devices grows, particularly with advancements in technology and an aging population, the opportunities for skilled engineers in this field are expanding.
In conclusion, for those aspiring to make a meaningful impact in healthcare technology, a commitment to continuous learning and skill development is paramount. By equipping themselves with the necessary qualifications and embracing innovative practices, aspiring engineers can position themselves for success in the flourishing medical device market. The future of healthcare technology is bright, and the role of product development engineers is crucial in shaping that future.
Frequently Asked Questions
What educational background is typically required to become a product development engineer in the medical device sector?
A bachelor’s degree in engineering—such as mechanical, biomedical, or electrical engineering—or a closely related field is typically required. Pursuing advanced degrees, like a Master’s in Biomedical Engineering, can enhance career opportunities.
What essential skills should a product development engineer in medical devices cultivate?
Essential skills include technical proficiency in CAD and simulation software, analytical skills for data analysis, project management experience, strong communication abilities, regulatory knowledge of FDA standards, creativity for problem-solving, and teamwork skills for collaboration.
How can hands-on experience benefit aspiring product development engineers?
Acquiring hands-on experience through internships or cooperative education programs provides practical insights and skills that are highly valued in the industry, making candidates more attractive to employers.
What steps can one take to navigate a career as a product development engineer in the medical device sector?
Steps include obtaining relevant education, gaining experience through internships, developing technical skills, networking with professionals, seeking certifications, applying for positions, preparing for interviews, and continuously improving by staying informed about industry trends.
What is the job outlook for product development engineers in the medical device field?
The Bureau of Labor Statistics forecasts a 20% increase in demand for product development engineers in medical devices through 2022, indicating robust opportunities in this sector.
Why is networking important for product development engineers in the medical device field?
Networking helps establish valuable connections, can lead to mentorship opportunities, and is crucial for career advancement, as emphasized by industry professionals.
What should candidates do to prepare for job applications and interviews in this field?
Candidates should actively look for job openings, customize their resumes to highlight relevant experiences, practice common interview questions, and be prepared to discuss their technical skills and relevant projects comprehensively.
How can continuous education impact a product development engineer's career?
Ongoing education is crucial for mastering relevant software and tools, ensuring that engineers remain competitive as technology evolves rapidly in the healthcare equipment sector.




