Overview
The main question addressed in the article is how to effectively choose a Contract Research Organization (CRO) for medical device trials in Argentina. The article outlines key criteria for selection, emphasizing the importance of a CRO’s experience with medical devices, regulatory expertise, quality assurance processes, and effective communication, which collectively enhance the likelihood of successful clinical studies in a complex regulatory environment.
Introduction
Navigating the regulatory landscape for medical device trials in Argentina presents unique challenges and opportunities for stakeholders in the healthcare sector. The National Administration of Drugs, Food and Medical Technology (ANMAT) plays a pivotal role in overseeing the registration and management of these trials, necessitating a comprehensive understanding of the associated regulations.
With projections indicating significant growth in the medical device regulatory affairs market, it becomes increasingly vital for organizations to adeptly maneuver through:
- Stringent requirements
- Ethics committee approvals
- Local laws
Furthermore, selecting the right Contract Research Organization (CRO) is crucial, as their expertise can significantly influence the success of clinical trials.
This article delves into the essential criteria for choosing a CRO, evaluating their performance, and fostering collaborative relationships, all aimed at ensuring effective and compliant medical device trials in Argentina's evolving healthcare landscape.
Navigating Argentina's Regulatory Landscape for Medical Device Trials
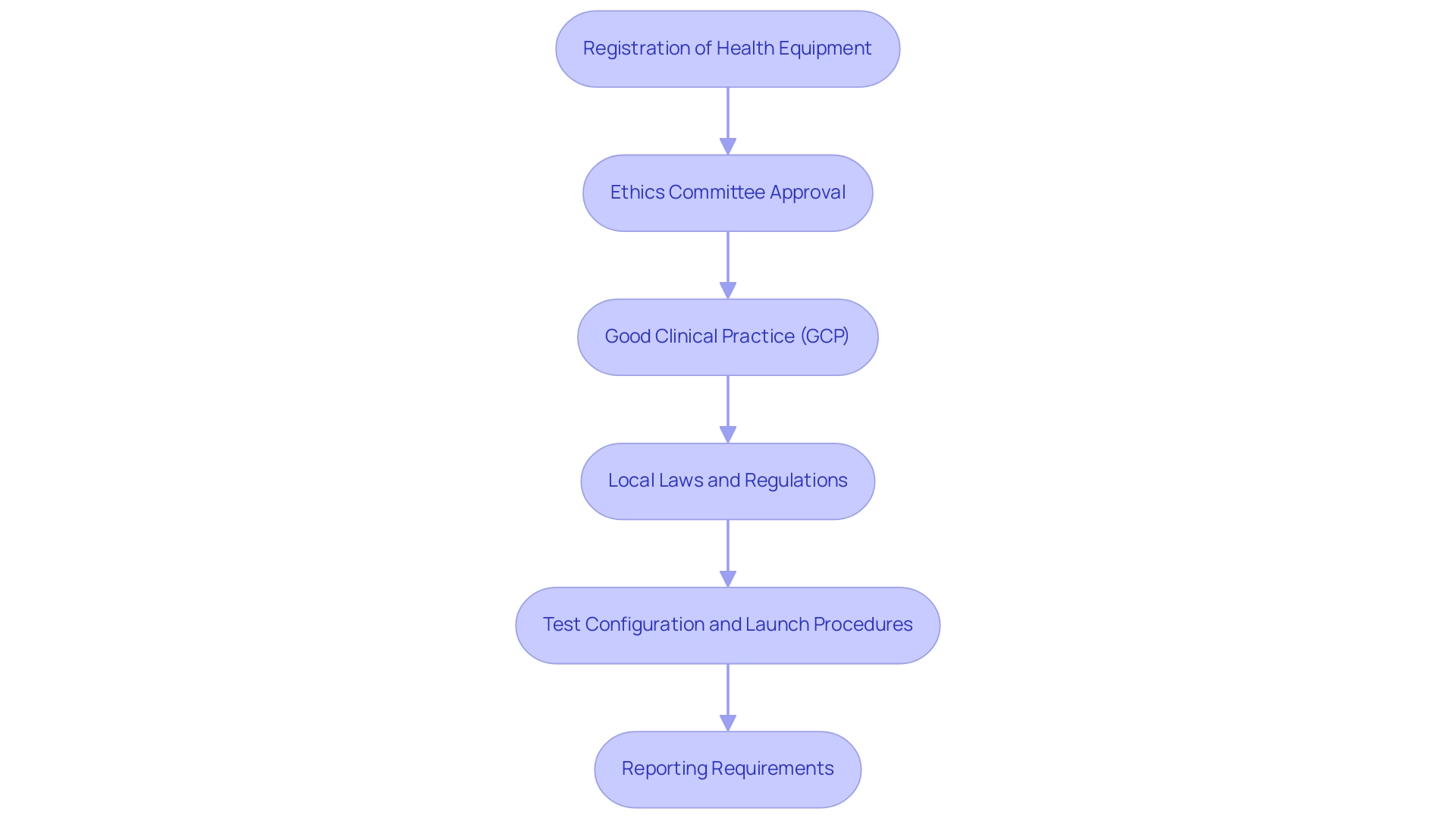
Argentina's regulatory structure for health product evaluations is primarily supervised by the National Administration of Drugs, Food and Medical Technology (ANMAT). Knowledge of the following regulations is essential for successful management:
-
Registration of Health Equipment: Before starting tests, it is essential that the health equipment is registered with ANMAT.
This process requires the submission of a comprehensive dossier, which should encompass technical documentation and relevant clinical data. Statistics indicate that this segment is expected to contribute significantly to the projected revenue growth of the medical device Regulatory Affairs market, estimated to reach US$ 99.0 million by 2030, with a compound annual growth rate (CAGR) of 9% anticipated from 2024 to 2030. However, stakeholders must navigate a challenging landscape characterized by strict currency controls, high inflation, and low foreign investment, complicating the registration process and overall market entry.
-
Ethics Committee Approval: All clinical studies must obtain approval from a recognized ethics committee. This requirement safeguards the ethical standards of the study and upholds the rights of participants, a critical aspect in the increasingly regulated market.
-
Good Clinical Practice (GCP): Adherence to GCP guidelines is essential.
These guidelines outline the responsibilities of the Contract Research Organization (CRO) and the sponsor, ensuring the protection of study participants and the integrity of data collected.
-
Local Laws and Regulations: It is vital to stay apprised of any regional legislation that may impact clinical studies, as these regulations can differ across provinces in Argentina.
-
Test Configuration and Launch Procedures: It is essential to understand the detailed processes involved in test configuration and launch, including obtaining necessary approvals from health ministries, which are crucial for compliance and successful initiation.
-
Reporting Requirements: Thorough reporting on study status, inventory, and the management of serious and non-serious adverse events is essential for ensuring transparency and accountability throughout the research process.
In addition to these regulatory requirements, insights from the databook reveal significant trends and company profiles within the Argentina healthcare validation and verification market. Comprehending these dynamics is essential for assessing potential CROs for Medical Device Trials Argentina regarding their expertise and compliance, as well as for improving the effectiveness of the process. As the healthcare industry expands and the demand for healthcare instruments increases, ensuring compliance with these guidelines will be crucial for CRO for Medical Device Trials Argentina in navigating the intricacies of clinical research.
The case study titled 'Argentina Healthcare Instrument Regulatory Affairs Market Outlook' emphasizes that the regulatory affairs market for healthcare instruments in Argentina is expected to grow, fueled by the expanding healthcare sector, rising healthcare needs, and an aging population, indicating a heightened demand for healthcare instruments and the necessity for regulatory approvals.
BOOK A MEETING to discuss how our services can assist you in successfully navigating these challenges.
Key Criteria for Choosing the Right CRO for Your Trials
When choosing a Contract Research Organization (CRO) for medical device trials in Argentina, it is crucial to consider the following key criteria:
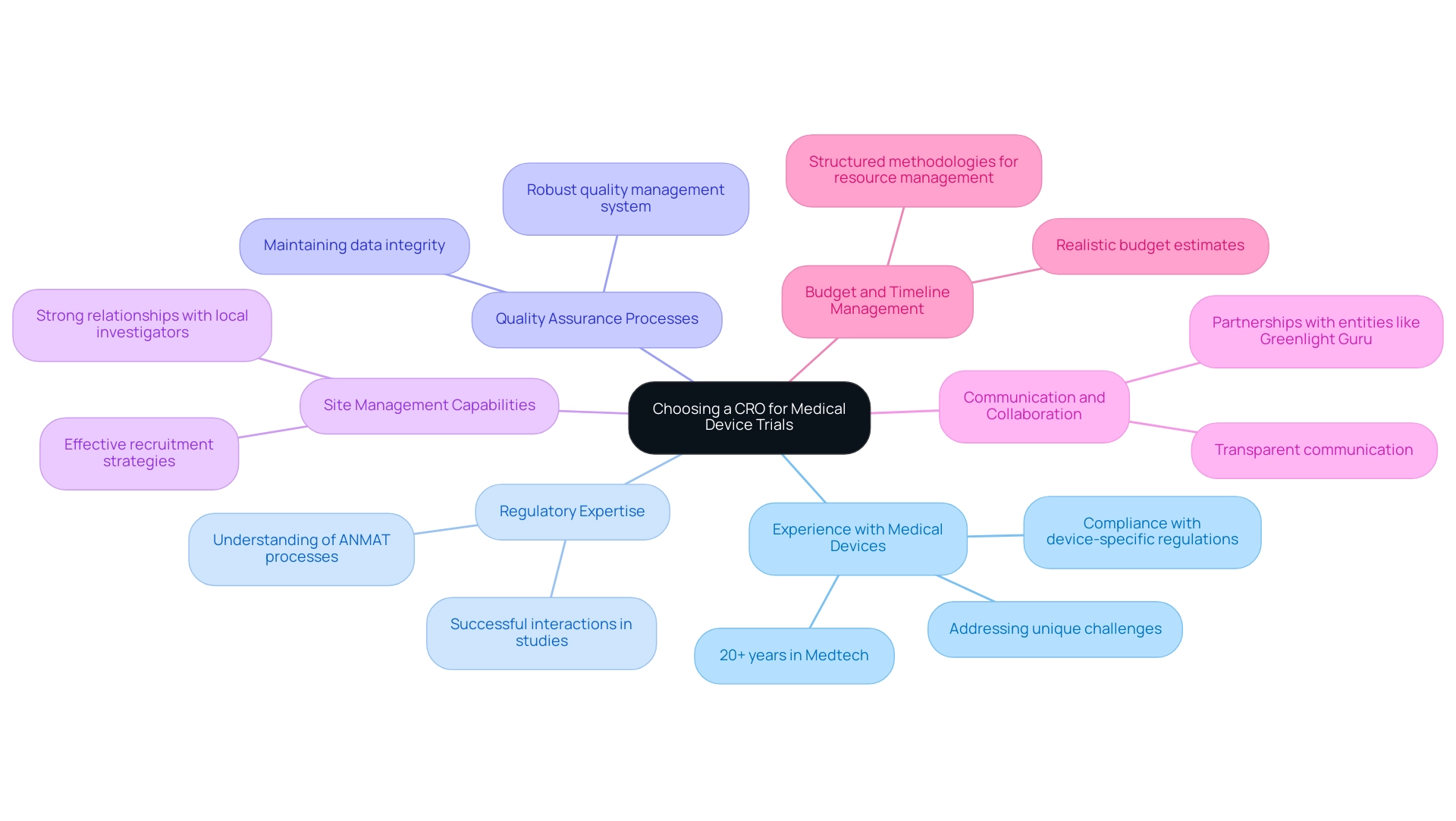
- Experience with Medical Devices: It is essential to choose a CRO with a proven history in managing medical device studies. bioaccess® offers over 20 years of specialized knowledge in Medtech, ensuring compliance with device-specific regulations and addressing the unique challenges they present.
- Regulatory Expertise: The CRO must possess a comprehensive understanding of Argentina's regulatory landscape, particularly the nuances of navigating ANMAT processes. bioaccess® has shown successful interactions in various studies, indicating their capability in this area.
- Quality Assurance Processes: A robust quality management system is essential for ensuring compliance and maintaining data integrity throughout the study. Evaluate the CRO’s quality assurance mechanisms; bioaccess® upholds the highest standards through meticulous processes.
- Site Management Capabilities: Effective management of clinical sites is critical. Assess the CRO’s strategies for recruitment, site monitoring, and its relationships with local investigators. bioaccess® has built robust relationships with local researchers, directly impacting study efficiency.
- Communication and Collaboration: Opt for a CRO that prioritizes transparent communication and collaboration. This approach fosters smoother interactions and significantly enhances the overall experience. bioaccess® emphasizes strong partnerships, as evidenced by collaborations with entities like Greenlight Guru, to advance clinical research initiatives.
- Budget and Timeline Management: The CRO should be able to provide realistic budget estimates and timelines. Their ability to manage resources effectively is essential to the success of the experiment. bioaccess® ensures efficient study execution through structured methodologies, such as detailed project planning and real-time budget tracking, which are essential for meeting both financial and temporal goals.
In 2023, Phase III studies constituted the largest portion of healthcare equipment assessments, with a revenue share of 53.18%, highlighting the significance of choosing a competent CRO. By meticulously evaluating these criteria, you can identify a CRO for Medical Device Trials Argentina, such as bioaccess®, that aligns with your specific requirements, ultimately enhancing the likelihood of successful medical device assessments. As Michael Baumann noted, "optimizing research methodologies could result in substantial financial benefits for biopharmaceutical companies, evidenced by potential double-digit improvements in net present value."
Bioaccess® enhances study efficiency through strong relationships with regulatory bodies and a commitment to innovation, making it a compelling choice for CRO selection.
Evaluating CRO Performance and Capabilities
To effectively assess the performance and capabilities of Contract Research Organizations (CROs), it is essential to consider several critical factors:
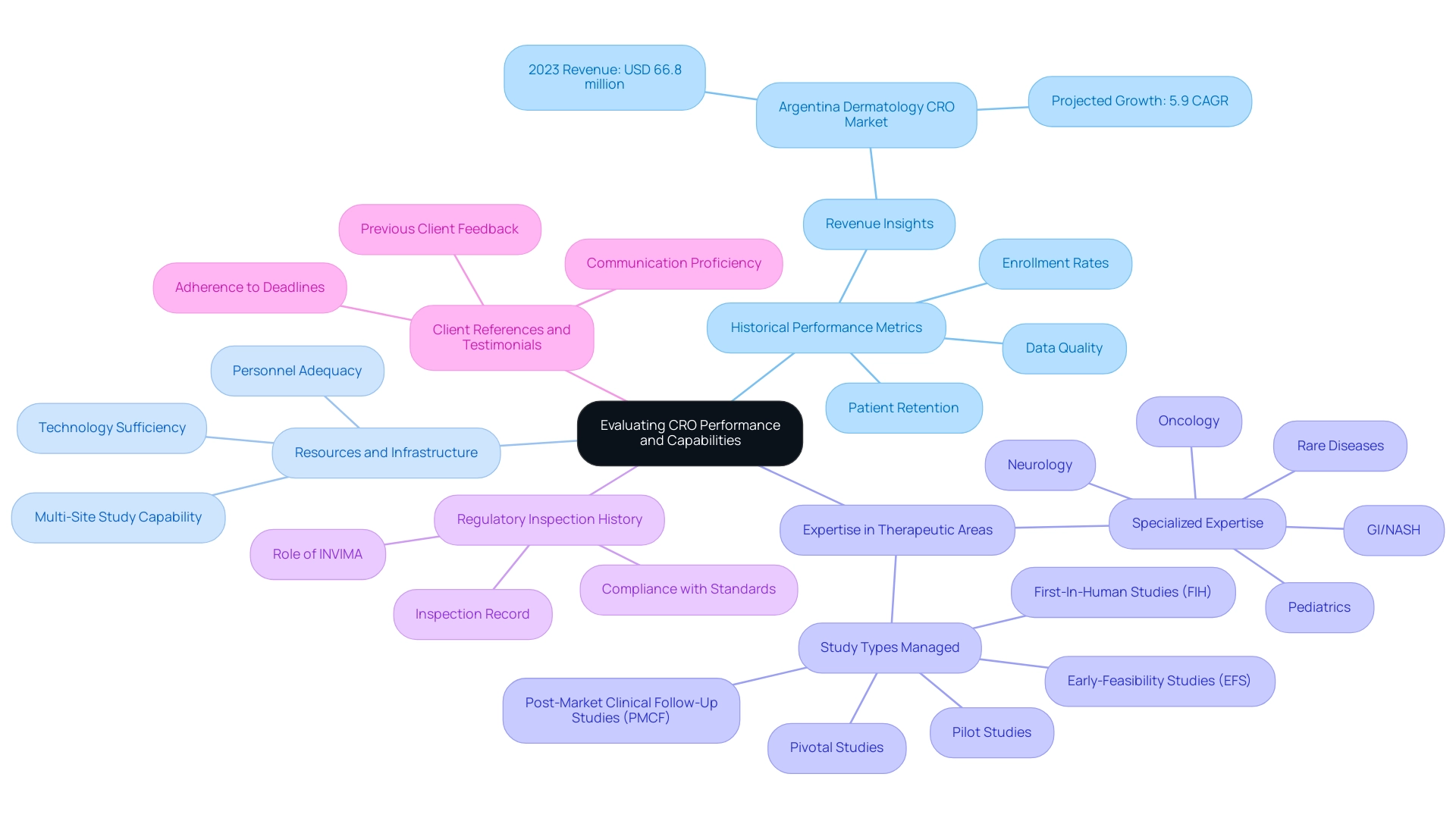
- Historical Performance Metrics: Analyze the CRO's track record, particularly in terms of completing studies on schedule and within budget. Key metrics to explore include enrollment rates, patient retention figures, and overall data quality. For instance, the Argentina dermatology CRO market generated a revenue of USD 66.8 million in 2023, with expectations to grow at a 5.9% CAGR through 2030. This growth is indicative of the potential for CRO for Medical Device Trials Argentina, especially since the clinical segment was the largest revenue generator in 2023, while the preclinical segment is anticipated to experience the fastest growth during the forecast period. Such performance indicators can provide valuable context for evaluating a CRO’s reliability.
- Resources and Infrastructure: Assess the sufficiency of the CRO's resources, including technology, personnel, and facilities essential to support your study requirements. This evaluation should also include their ability to handle multi-site studies, which is increasingly important in today’s research environment. Robust infrastructure is essential for guaranteeing efficient execution and data management.
- Expertise in Therapeutic Areas: Confirm that the CRO possesses specialized expertise in the relevant therapeutic areas associated with your medical device. With specialized expertise across 14 therapeutic centers of excellence, such as oncology, GI/NASH, pediatrics, neurology, and rare diseases, a CRO for Medical Device Trials Argentina, like bioaccess®, with over 20 years in Medtech, can significantly improve the quality of both study design and execution. Bioaccess® specializes in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
- Regulatory Inspection History: Investigate the CRO's history concerning regulatory inspections. A flawless inspection record generally indicates a strong dedication to adherence and quality standards, which is essential for upholding the integrity of clinical studies. Grasping the function of INVIMA, Colombia's National Food and Drug Surveillance Institute, can enhance your assessment, as it offers supervision and categorizations pertinent to health products.
- Client References and Testimonials: Solicit references from previous clients to gain insights into the CRO's overall performance, communication proficiency, and ability to adhere to deadlines. This feedback can be instrumental in forming a well-rounded view of the CRO's operational capabilities.
As Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes, "Get in touch with us now" to ensure you have the right CRO partner for your needs. Bioaccess® provides a personalized method designed to fulfill the specific needs of your clinical studies.
By systematically assessing these factors, you can instill confidence in your choice of CRO, ensuring they are well-equipped to deliver successful clinical studies for your medical device.
Establishing a Collaborative Relationship with Your Chosen CRO
Once a Clinical Research Organization (CRO) has been chosen, fostering a cooperative relationship is crucial for the success of the study. Here are several strategies to facilitate effective collaboration:
-
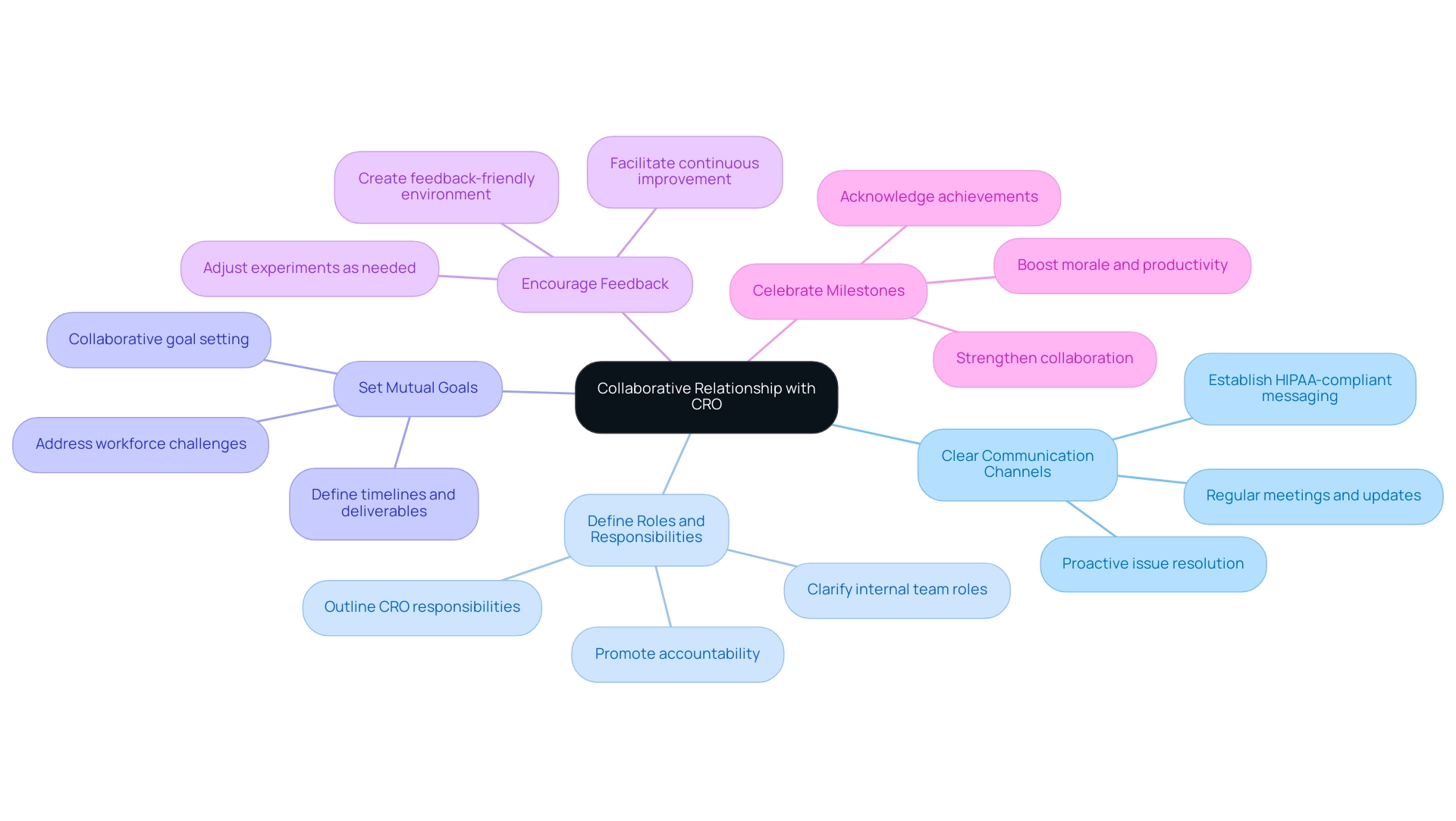
Clear Communication Channels: From the outset, ensure clear lines of communication are established.
Utilizing HIPAA-compliant text messaging templates can help ensure that all necessary information is included and conveyed effectively. Regular meetings and updates not only align expectations but also allow for timely resolution of any issues that may arise, fostering a proactive approach to collaboration.
-
Define Roles and Responsibilities: It is critical to delineate the roles and responsibilities of both your internal team and the CRO.
As Kelly Conklin, Chief Clinical Officer, notes, organizations are increasingly looking at evolving roles to optimize collaboration. This clarity minimizes the risk of misunderstandings and promotes accountability, ultimately enhancing workflow efficiency.
-
Set Mutual Goals: Collaboratively establishing goals for the trial—including timelines and deliverables—instills a sense of partnership and commitment.
Common goals drive focus and enhance overall performance, which is increasingly vital given the challenges posed by staff shortages and clinician burnout in the healthcare sector, with estimates suggesting a 20% loss of the workforce since the pandemic.
-
Encourage Feedback: Create an environment where open and constructive feedback is encouraged. This not only leads to continuous improvement but also strengthens the collaborative process, allowing both parties to adjust and enhance the experiment as needed.
-
Celebrate Milestones: Acknowledging and celebrating achievements throughout the process is important. Acknowledging milestones not only encourages motivation but also strengthens the collaboration, boosting both morale and productivity.
By applying these strategies and utilizing the extensive service capabilities of your CRO for Medical Device Trials Argentina—such as feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and detailed reporting on study status, inventory, and adverse events—you can nurture a productive and cooperative relationship, significantly enhancing the chances of a successful clinical study. These efforts not only enhance study outcomes but also contribute positively to local economies by creating jobs and fostering economic growth, highlighting the broader impact of medtech clinical studies.
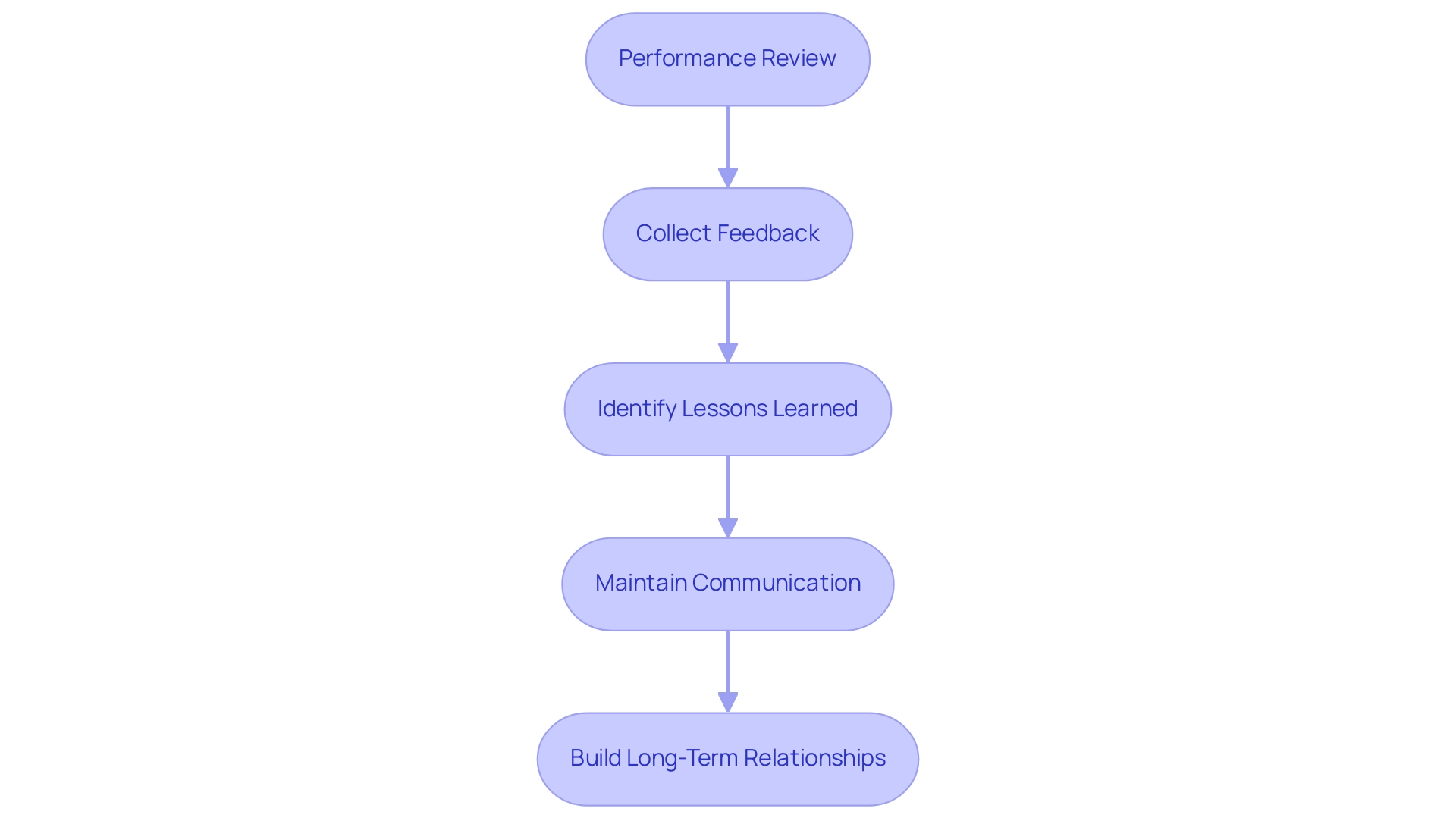
Post-Trial Evaluation and Relationship Management
Upon completion of your medical device study, conducting a comprehensive evaluation of the CRO for Medical Device Trials Argentina's performance is essential for future success. Here are key steps to guide this evaluation:
- Performance Review: Begin by assessing the CRO’s performance against the metrics and goals established at the outset. Assessing factors like efficiency, data quality, and compliance with timelines offers a clear view of their effectiveness in overseeing the process. This can be particularly important in a regulatory environment like Colombia, where adherence to INVIMA's standards is crucial. Additionally, consider their effectiveness in feasibility and selection of research site and principal investigator (PI) as part of this review.
- Collect Feedback: Engage your team and stakeholders who took part in the experiment to gather their insights. This collaborative feedback can illuminate the CRO’s strengths and highlight areas needing improvement, ensuring a well-rounded evaluation.
- Identify Lessons Learned: Documenting lessons learned throughout the process is crucial. Understanding what strategies were successful and which challenges arose can significantly inform future projects and aid in the selection of CROs. As noted in a recent survey conducted by the German Association of Research-Based Pharmaceutical Companies, 81% of respondents reported having a Standard Operating Procedure (SOP) or guiding document, with 94% of these deemed global. Such frameworks can enhance lesson identification and ensure that oversight processes maintain high quality and integrity of data in outsourced clinical studies. It is also essential to review how the CRO managed reporting on study status and adverse events during the investigation.
- Maintain Communication: Keep communication channels open with the CRO after the study concludes. Discussing evaluation findings fosters transparency and may lead to discovering opportunities for future collaboration. Michael Hennig from GlaxoSmithKline GmbH & Co. KG emphasizes the importance of transparent communication, stating, "Finally, the guiding principles for all oversight processes should be transparent communication, a clearly established expectation for quality, a precise definition of accountability and responsibility while avoiding silo mentality, and a comprehensive documentation of the oversight’s evidence."
- Build Long-Term Relationships: If the CRO has demonstrated commendable performance, consider developing a long-term partnership for upcoming trials. Strong relationships can enhance collaboration and improve efficiency in future projects, ultimately contributing to the integrity and quality of clinical research, especially in CRO for Medical Device Trials Argentina. Given the importance of compliance with local regulatory standards, such as those mandated by INVIMA, fostering these relationships can be particularly beneficial for U.S. medical device companies operating in Colombia.
By implementing these steps in your post-trial evaluation, you can not only enhance your subsequent clinical research endeavors but also ensure continuous improvement in your relationships with CROs, thereby leveraging their expertise in navigating complex regulatory landscapes.
Conclusion
Navigating the complexities of medical device trials in Argentina requires a nuanced understanding of the regulatory landscape, particularly the role of the National Administration of Drugs, Food and Medical Technology (ANMAT). Key considerations include:
- The registration of medical devices
- Ethics committee approvals
- Adherence to Good Clinical Practice (GCP)
- Compliance with local laws
Each of these elements plays a critical role in ensuring the success of clinical trials, as they safeguard participant rights and maintain the integrity of the data collected.
Selecting the right Contract Research Organization (CRO) is equally important. Evaluating potential CROs based on their:
- Experience with medical devices
- Regulatory expertise
- Quality assurance processes
can significantly influence trial outcomes. Establishing a collaborative relationship with the chosen CRO further enhances the likelihood of success. Clear communication, defined roles, mutual goals, and constructive feedback are essential strategies for fostering this partnership.
Ultimately, the growth of the medical device regulatory affairs market in Argentina presents both challenges and opportunities. By understanding the regulatory requirements and strategically partnering with a competent CRO, stakeholders can navigate the complexities of clinical trials more effectively. This comprehensive approach will not only ensure compliance and efficiency but also contribute to the advancement of healthcare solutions in an evolving market.
Frequently Asked Questions
What is the main regulatory body overseeing health product evaluations in Argentina?
The National Administration of Drugs, Food and Medical Technology (ANMAT) supervises the regulatory structure for health product evaluations in Argentina.
What is required for the registration of health equipment in Argentina?
Health equipment must be registered with ANMAT before testing begins. This registration requires a comprehensive dossier that includes technical documentation and relevant clinical data.
What challenges do stakeholders face during the registration process in Argentina?
Stakeholders must navigate a challenging landscape characterized by strict currency controls, high inflation, and low foreign investment, which complicates the registration process and overall market entry.
Is ethics committee approval necessary for clinical studies in Argentina?
Yes, all clinical studies must obtain approval from a recognized ethics committee to safeguard ethical standards and uphold participant rights.
What guidelines must be followed to ensure Good Clinical Practice (GCP)?
Adherence to GCP guidelines is essential, outlining the responsibilities of the Contract Research Organization (CRO) and the sponsor to protect study participants and ensure data integrity.
Why is it important to be aware of local laws and regulations in Argentina?
It is vital to stay informed about regional legislation that may impact clinical studies, as regulations can differ across provinces in Argentina.
What processes are involved in test configuration and launch procedures?
Understanding the detailed processes for test configuration and launch is essential, including obtaining necessary approvals from health ministries for compliance and successful initiation.
What are the reporting requirements during clinical studies?
Thorough reporting on study status, inventory, and management of serious and non-serious adverse events is essential for ensuring transparency and accountability throughout the research process.
What key criteria should be considered when choosing a CRO for medical device trials in Argentina?
Key criteria include experience with medical devices, regulatory expertise, quality assurance processes, site management capabilities, communication and collaboration, and budget and timeline management.
How does bioaccess® stand out as a CRO for medical device trials in Argentina?
Bioaccess® has over 20 years of specialized knowledge in Medtech, strong relationships with local researchers, and a commitment to quality assurance and efficient study execution, making it a compelling choice for CRO selection.




