Overview
Designing clinical trials for medical devices in Argentina requires a structured approach, encompassing phases such as:
- Feasibility assessments
- Pilot trials
- Pivotal trials
- Post-market evaluations
All while strictly adhering to ANMAT regulations. Understanding local regulatory guidelines is paramount, as is the implementation of effective participant recruitment strategies to ensure successful trial outcomes. Organizations like bioaccess® play a critical role in facilitating these processes, addressing key challenges within the Medtech landscape. Their expertise not only reinforces the importance of collaboration but also underscores the next steps that stakeholders must consider to navigate this complex environment.
Introduction
In the realm of medical innovation, clinical trials for medical devices serve as a pivotal gateway to ensuring safety and efficacy before products reach the market. These trials are meticulously structured and governed by regulatory frameworks, unfolding through various critical phases—each designed to address distinct challenges and requirements. In Latin America, where unique geographical and cultural factors come into play, understanding these phases is essential for stakeholders aiming to navigate the complexities of clinical research.
From feasibility studies that assess trial practicality to post-market evaluations that monitor long-term safety, the journey of a medical device is fraught with both obstacles and opportunities.
As organizations like bioaccess® step in to offer tailored solutions, the landscape of clinical trials continues to evolve, promising advancements that could significantly enhance patient outcomes and foster local economic growth.
Understanding Clinical Trials for Medical Devices
Clinical studies for medical devices represent meticulously structured investigations aimed at evaluating the safety and effectiveness of innovative technologies. These tests generally progress through several essential stages: preclinical evaluations, pilot experiments, pivotal assessments, and post-market reviews. Grasping these phases is vital for stakeholders engaged in the development and approval of medical devices, particularly in Latin America, where bioaccess® provides expedited clinical research services tailored to address these requirements.
Feasibility Assessments: These preliminary evaluations examine the viability of conducting a test, encompassing patient recruitment and site selection. Given that approximately 70% of the population in Latin America lives two hours or more from an academic Medical Center, strategic planning for patient access is crucial. This geographical challenge underscores the importance of effective recruitment strategies to ensure adequate participant enrollment, a focus area for bioaccess®.
Pilot Trials: These smaller-scale experiments concentrate on initial safety and effectiveness, enabling researchers to refine protocols prior to larger investigations. Bioaccess® specializes in overseeing these assessments to ensure they are conducted efficiently and effectively.
Pivotal Trials: These large-scale investigations are designed to provide definitive evidence of a device's effectiveness and safety. They are essential for regulatory approval and must adhere to stringent standards. With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate these complex trials successfully.
Post-Market Clinical Follow-Up Assessments (PMCF): After a device is available in the market, PMCF assessments track long-term safety and effectiveness, ensuring continuous adherence to regulatory requirements. Bioaccess® guarantees that these studies are conducted with the utmost rigor, contributing to the sustained success of medical devices in the market.
Each phase serves a distinct purpose, from initial safety assessments to comprehensive efficacy evaluations, ensuring that devices meet regulatory standards before reaching the market. For instance, addressing the issue of screen failures—averaging around $1,200 per failure—can significantly enhance testing efficiency and reduce costs, ultimately improving success rates. This emphasizes the essential requirement for efficient patient selection and recruitment methods, especially in a region where logistical obstacles can hinder study advancement.
Recent data indicates a rising number of studies performed for medical devices in Latin America, reflecting a greater acknowledgment of the region's capacity for groundbreaking research. However, concerns persist regarding the impact of data banks on patent rights and the risk of offshore research, which could jeopardize the competitive edge of small manufacturers. Stakeholders highlight the significance of safeguarding proprietary information, as confidentiality is vital for promoting innovation and ensuring adherence to regulatory standards in research studies.
Understanding these concepts and the existing regulatory environment, including the supervision provided by INVIMA as a Level 4 health authority, is essential when designing clinical trials for medical devices in Argentina. This ultimately facilitates the advancement of medical devices that can significantly improve patient outcomes while contributing to local economic growth through job creation and international collaboration. Bioaccess®'s customized approach ensures that each study is tailored to meet the unique challenges of the region, further enhancing the likelihood of successful outcomes.
Navigating the Regulatory Framework in Argentina
In Argentina, the design of clinical trials for medical devices is governed by ANMAT (Administración Nacional de Medicamentos, Alimentos y Tecnologia Medica). This agency is responsible for overseeing the approval process related to these trials, ensuring compliance with established safety and ethical standards. Researchers must submit a comprehensive clinical study dossier that includes the study protocol, informed consent forms, and detailed safety data.
Familiarity with ANMAT's guidelines is crucial for the design of clinical trials for medical devices in Argentina. This includes obtaining ethical committee approval and adhering to Good Clinical Practice (GCP) standards, which are vital for maintaining the integrity of the research process. Additionally, understanding the categorization of medical devices by risk levels—Class I, II, and III—is essential, as it directly influences the specific regulatory requirements applicable to each study.
bioaccess® offers a comprehensive approach to enhancing medical device evaluations in Latin America, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting on study status and adverse events
As highlighted by Mercedes Ponce de Leon, Senior Manager in Regulatory Affairs, "Designing clinical trials for medical devices in Argentina requires a thorough understanding of ANMAT's evolving guidelines and a proactive approach to compliance." Engaging with local regulatory experts can significantly streamline the navigation of the processes involved in designing clinical trials for medical devices in Argentina.
Their insights into the nuances of ANMAT regulations and the current landscape of clinical trials can provide invaluable assistance, ensuring compliance and enhancing the likelihood of successful outcomes. Leveraging bioaccess®'s expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies can further bolster the success of research in this region.
BOOK A MEETING.
Strategies for Effective Participant Recruitment
Recruiting participants for clinical studies in Argentina presents unique challenges due to the country's diverse population. To optimize recruitment efforts, researchers should consider the following strategies:
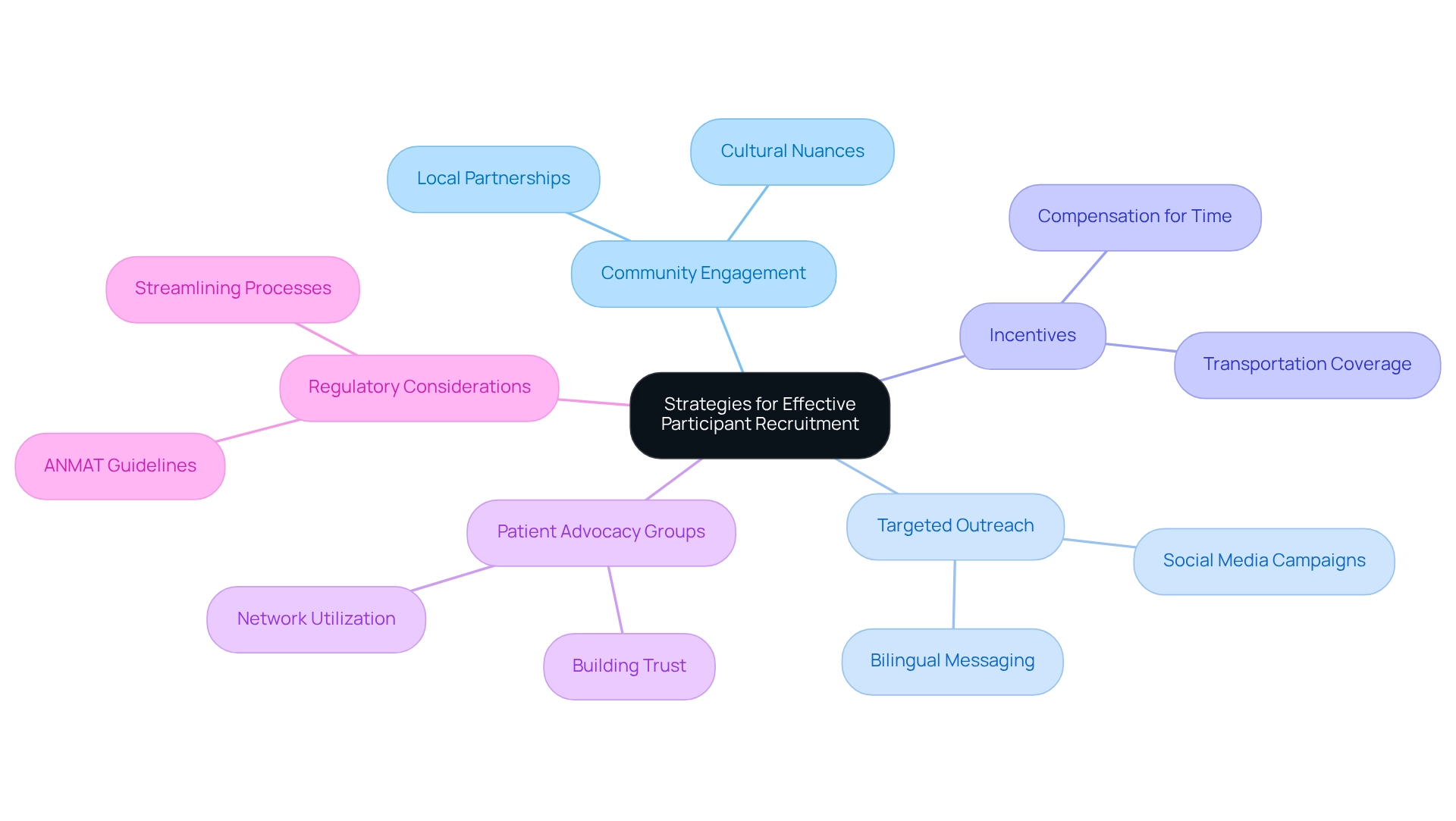
- Community Engagement: Establish partnerships with local healthcare providers and community organizations to foster awareness about the study and its potential benefits. Involving the community not only fosters trust but also promotes participation by emphasizing the study's significance to local health concerns. As noted by Septem Riza et al., effective community engagement is crucial for successful recruitment. This approach is particularly important in Latin America, where understanding cultural nuances can significantly impact recruitment success.
- Targeted Outreach: Leverage social media platforms and local advertising to effectively reach specific demographics that align with the trial's eligibility criteria. Tailoring messages to resonate with different community segments can significantly enhance visibility and interest. Given the growing prevalence of Spanish in the U.S. and its cultural significance, utilizing bilingual outreach can further improve engagement with potential participants.
- Incentives: Offering compensation for individuals' time and travel can significantly enhance enrollment rates. Financial incentives, along with covering transportation expenses, show appreciation for contributors' efforts and can reduce obstacles to involvement. Given that it takes an average of 10 to 15 years to develop a new drug, efficient recruitment strategies are essential to streamline the process. This is especially relevant in the context of rising drug development costs, as highlighted in recent studies.
- Patient Advocacy Groups: Work together with advocacy organizations to reach out to potential individuals who might gain from the study. These groups frequently have built trust within the community and can assist with introductions to individuals who satisfy the study criteria. By leveraging these networks, researchers can enhance their outreach efforts and improve recruitment outcomes.
- Regulatory Considerations: Understanding the regulatory environment is crucial for recruitment strategies. Argentina's drug regulatory body (ANMAT) is acknowledged as a regional reference authority, and its guidelines should guide recruitment methods to ensure adherence and facilitate smoother processes.
By applying these strategies, researchers can improve participant recruitment and retention rates, ultimately aiding the success of designing clinical trials for medical devices in Argentina. Moreover, as bioaccess® remains at the forefront of designing clinical trials for medical devices in Argentina, the incorporation of extensive study management services—such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—will be crucial in navigating the intricacies of the region's Medtech environment.
Step-by-Step Process for Designing Clinical Trials
Designing clinical trials for medical devices in Argentina is a meticulous process that demands careful planning and execution. A structured approach is essential for ensuring a successful trial:
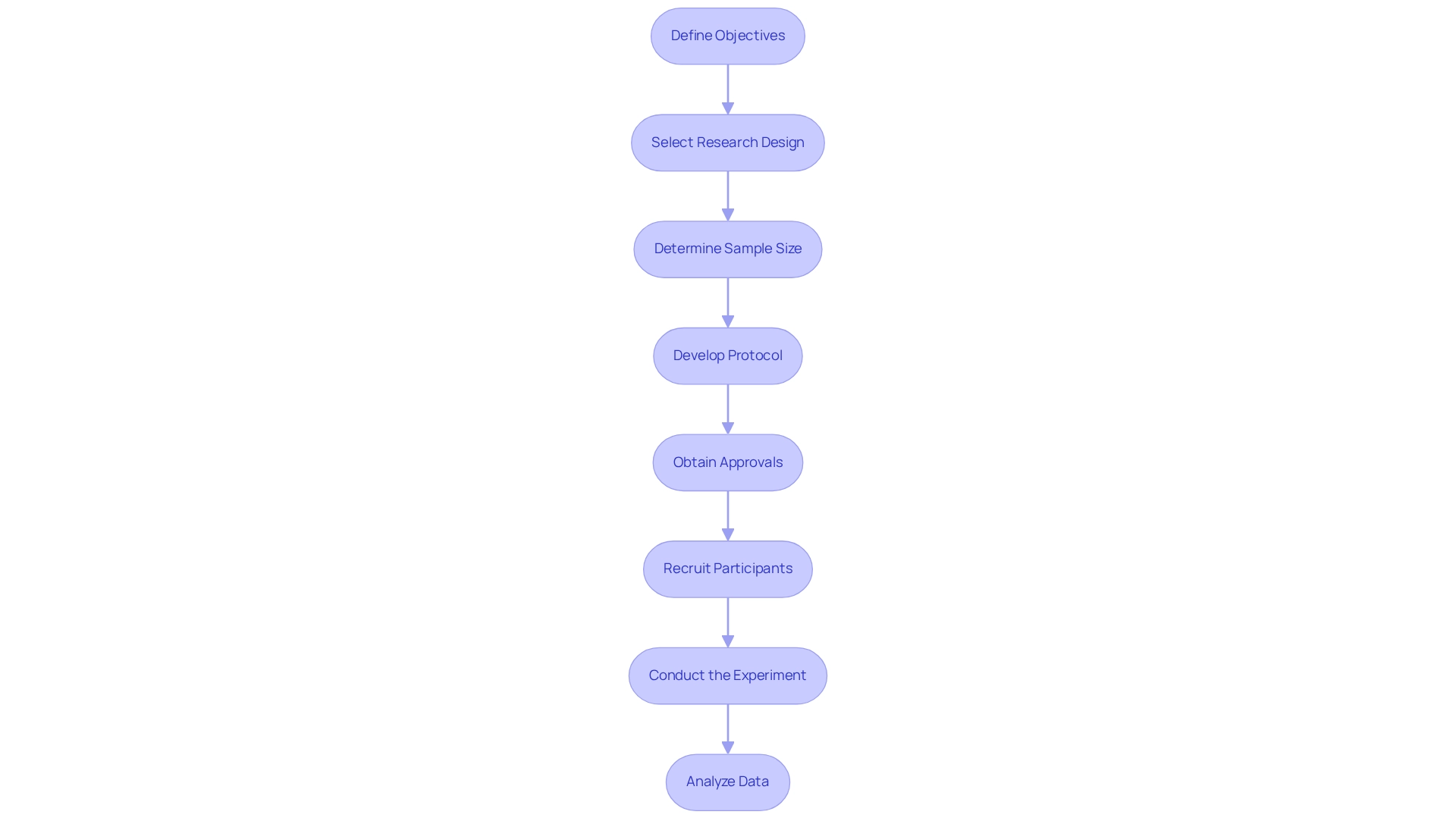
- Define Objectives: Begin by clearly outlining the primary and secondary goals of the study. This step is crucial as it directs the entire research and aids in measuring outcomes effectively.
- Select Research Design: Choose a suitable research approach, such as a randomized controlled experiment (RCT) or an observational analysis, based on the defined objectives. The selection of design greatly influences the validity and applicability of the study results.
- Determine Sample Size: Calculate the necessary sample size to ensure statistical validity. This involves considering the expected effect size, variability, and the desired power of the study. A well-structured study should aim for a sample size that can identify clinically significant differences while minimizing expenses and resources.
- Develop Protocol: Create a comprehensive protocol that details the methodology, endpoints, and data collection methods. A robust protocol acts as a roadmap for the study, ensuring consistency and adherence to regulatory standards.
- Obtain Approvals: Secure the necessary approvals from regulatory bodies and ethics committees. In Latin America, this includes navigating the regulatory landscape governed by entities such as INVIMA in Colombia, which oversees medical device classification and compliance as a Level 4 health authority by PAHO/WHO. This step is essential to ensure that the examination complies with ethical standards and regulatory requirements, which can differ greatly across regions.
- Recruit Participants: Implement effective recruitment strategies to enroll participants. Consideration of the target population's characteristics and potential barriers to participation is essential for achieving adequate enrollment.
- Conduct the Experiment: Execute the experiment according to the established protocol, ensuring compliance with regulatory standards throughout the process. This includes monitoring for safety and efficacy, as well as maintaining data integrity.
- Analyze Data: After completion, analyze the data to assess safety and efficacy. Integrating results from RCTs with observational data can enhance the applicability of findings to broader populations, addressing challenges in generalizing results to new contexts. Understanding the underlying causal structures and support factors is essential for effective application beyond the study context, as emphasized in a case study on the challenges in extrapolating RCT results.
Incorporating patient perspectives is also vital. As Steven Keating noted, "How come as a patient, we’re always last in line to the data?" This highlights the significance of ensuring that study designs consider patient access to information and the implications for their participation in the research process.
Furthermore, it is essential to recognize that a post-market surveillance plan must be submitted to the FDA as part of premarket approval applications, detailing activities tailored to the device's risks, intended use, and patient population. Current best practices highlight the importance of incorporating varied data sources and comprehending the distinct traits of the target population to improve the relevance and influence of research findings. For instance, the J-PAL research on CCT in Malawi reported an additional 0.09 and 0.07 years of education for every US$100 spent, illustrating the importance of resource allocation in achieving meaningful outcomes.
At bioaccess®, we utilize over 20 years of expertise in overseeing research initiatives in Latin America, providing extensive services that encompass feasibility assessments, site selection, compliance reviews, project setup, import permits, project management, and reporting. Our emphasis on early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies guarantees that your studies are not only compliant but also strategically positioned for success, particularly when designing clinical trials for medical devices in Argentina. We understand the unique challenges faced in this region, such as varying regulatory requirements and cultural considerations, and we are committed to providing tailored solutions that meet the needs of our clients.
Ethical Considerations in Clinical Trial Design
Ethical considerations are paramount in the design of clinical trials for medical devices in Argentina, particularly within the medical device sector. Key aspects include:
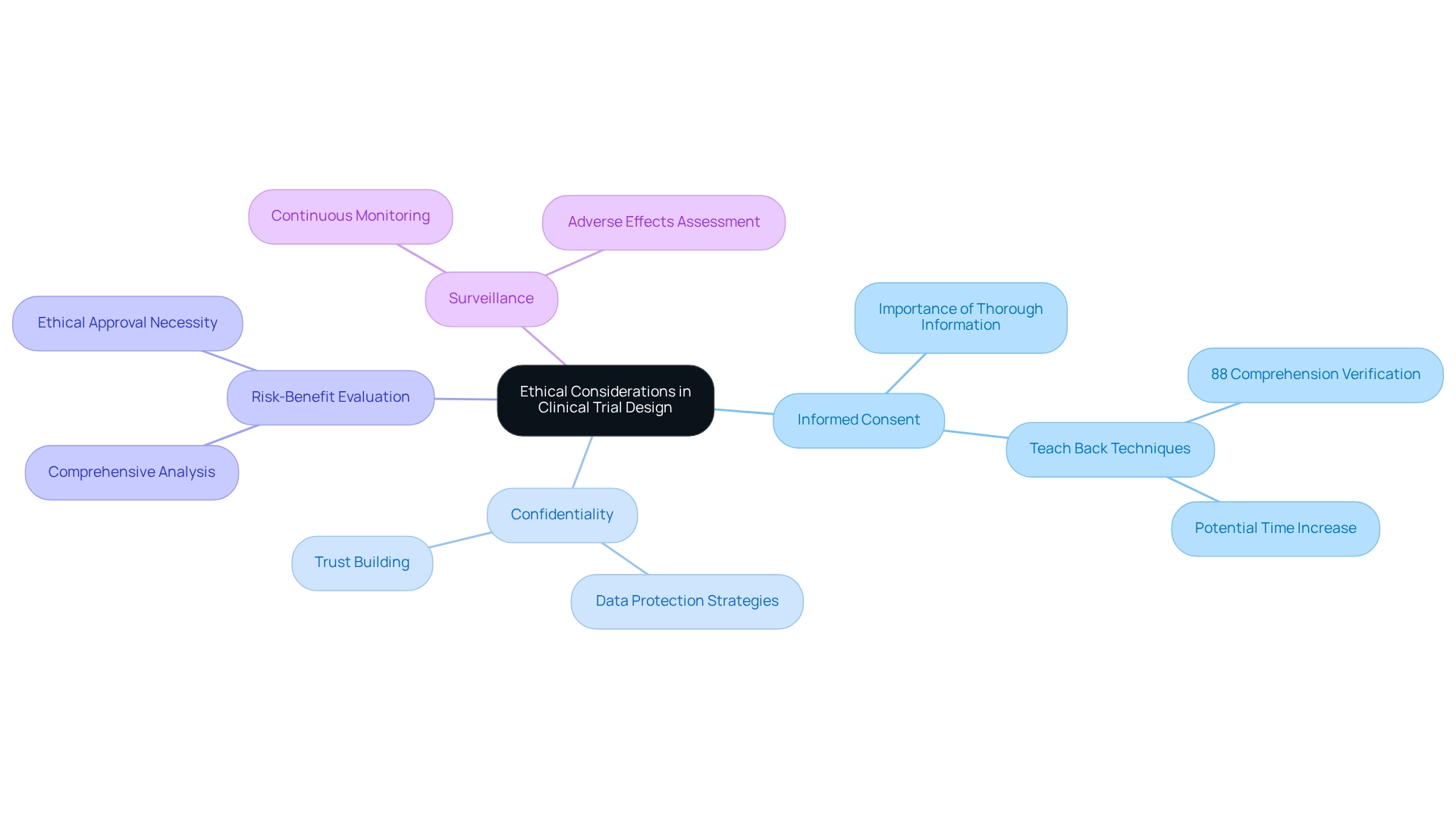
- Informed Consent: Thoroughly informing individuals about the study's purpose, procedures, risks, and benefits prior to enrollment is crucial. Effective informed consent processes not only respect individual autonomy but also enhance the integrity of the trial. A study highlighted the application of Teach Back techniques, confirming that while 88% of research personnel verified individuals' comprehension, employing these strategies could significantly improve communication, ensuring that individuals grasp essential information, albeit potentially increasing the time required for the process. This approach holds particular relevance in Latin America, where cultural nuances may impact understanding.
- Confidentiality: Safeguarding individuals' personal information is essential. This requires implementing robust data protection strategies during the study to shield confidential information from unauthorized access, thereby fostering trust between subjects and researchers. bioaccess® is dedicated to ensuring information security and has established grievance and data protection procedures to address any concerns that may arise, ensuring compliance and transparency in all processes. For any queries or concerns regarding the processing of your information, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
- Risk-Benefit Evaluation: A comprehensive risk-benefit evaluation is necessary to confirm that the potential advantages of the study outweigh the risks to participants. This analysis is vital for ethical approval, especially in the context of designing clinical trials for medical devices in Argentina, to ensure the safety and well-being of those involved.
- Surveillance: Continuous monitoring of subject safety and study integrity is essential. This includes regular assessments to identify any adverse effects or ethical issues that may arise during the study.
Adhering to these ethical standards not only protects those involved but also enhances the credibility of the research outcomes. As of 2025, the framework of ethical considerations in research studies continues to evolve, with a total of 921 device studies submitted as 'delayed posting' under the FDAAA, underscoring the importance of maintaining high ethical standards in medical research. As noted by research staff, "info leaflets are getting more complicated with GDPR/data protection information," making it nearly impossible to shorten them without risking rejection by the ethics committee. Furthermore, the largest study involved 100 individuals, while the smallest included 11, illustrating the varying scales of medical investigations conducted in the region. Data collection for relevant studies occurred between September 2020 and February 2021, utilizing both paper and electronic survey formats to maximize accessibility for participants.
bioaccess® specializes in overseeing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), ensuring a comprehensive approach to study management.
Collaborating with Local Stakeholders and Institutions
Cooperation with local stakeholders is essential for the successful design of clinical trials for medical devices in Argentina. To maximize the effectiveness of these partnerships, consider the following strategies:
- Engage Local Institutions: Form alliances with universities and research centers to leverage their expertise and resources. These institutions often possess established networks that can significantly enhance the design and implementation of the study.
- Build Relationships with Healthcare Providers: Establish robust connections with local physicians and hospitals. Their involvement is critical for participant recruitment and data collection, as they can facilitate access to potential study participants and ensure adherence to research protocols.
- Involve Community Leaders: Engaging community leaders fosters trust and encourages participation among local populations. Their support can alleviate concerns and highlight the benefits of the research study, leading to increased enrollment rates.
- Utilize Local CROs: Collaborate with local Contract Research Organizations (CROs) that possess an in-depth understanding of the regulatory landscape and cultural nuances. These organizations can assist with management of the assessment, ensuring compliance and enhancing the study's credibility.
The impact of local stakeholder involvement on the design of clinical trials for medical devices in Argentina is profound. Successful initiatives have shown that collaborations, particularly in this context, lead to equitable access to therapeutics, improved data collection for sponsors, and better healthcare outcomes. For instance, ReGelTec's Early Feasibility Study in Colombia, which effectively treated eleven patients with chronic low back pain using HYDRAFIL™, exemplifies how local engagement can shape impactful clinical research.
The study's design benefited from stakeholder feedback, effectively addressing logistical challenges and enhancing patient access to care.
Moreover, statistics indicate that effective cooperation between CROs and local organizations can significantly improve study results, particularly in the context of designing clinical trials for medical devices in Argentina, with a notable increase in participant retention and data quality. RadComp's newsletters report a 20% click-rate on content, suggesting that well-communicated strategies can enhance participation and interest in research studies. As the landscape of medical research evolves in 2025, the design of clinical trials for medical devices in Argentina will be crucial for emphasizing collaborations that advance medical devices and ensure that studies are both efficient and ethically conducted.
As Shea Hickey, Senior Clinical Research Coordinator, emphasizes, "Involving local stakeholders is not just advantageous; it is crucial for the integrity and success of research studies." This perspective highlights the importance of cultivating strong relationships within the community to achieve meaningful outcomes.
Leveraging Technology in Clinical Trials
Technology serves as a cornerstone of modern medical studies, significantly enhancing both efficiency and participant experience, particularly in the design of clinical trials for medical devices in Argentina. Key technologies to consider include:
- Electronic Data Capture (EDC): EDC systems streamline data collection and management, minimizing errors and boosting operational efficiency. Recent statistics indicate that the adoption of EDC in clinical studies has led to a reduction in data entry mistakes by up to 30%, underscoring its importance in preserving data integrity. Bioaccess® employs EDC systems in the design of clinical trials for medical devices in Argentina, ensuring high-quality data management across all study phases, from Early-Feasibility Studies to Post-Market Clinical Follow-Up Studies.
- Telemedicine: The emergence of telehealth solutions facilitates remote consultations and follow-ups, enhancing participant engagement and increasing convenience. This approach proves particularly beneficial in engaging diverse groups, making research studies more inclusive and accessible. As Carmen Rosa from the National Institutes of Health notes, "This crisis has also interrupted research operations in the U.S. and globally," highlighting the need for flexible solutions like telemedicine. Bioaccess® is dedicated to designing clinical trials for medical devices in Argentina by incorporating telemedicine into its clinical study management services, ensuring individuals can engage with research regardless of their location.
- Wearable Devices: The integration of wearable technology allows for real-time tracking of individuals' health metrics, providing invaluable data for analysis. This technology not only enhances data precision but also empowers individuals by keeping them informed about their health status during the study. Bioaccess® is committed to designing clinical trials for medical devices in Argentina by utilizing wearable devices to improve data collection and participant engagement in First-In-Human and Pivotal Studies.
- Data Analytics: Employing advanced data analytics tools enables researchers to interpret study data effectively, leading to more informed decision-making. With the research studies market projected to reach $4.9 billion by 2028, leveraging data analytics is essential for improving study outcomes and ensuring regulatory compliance. This growth illustrates the increasing reliance on technology in medical research. Bioaccess® utilizes advanced data analytics to generate insights and enhance study efficiency in the context of designing clinical trials for medical devices in Argentina, particularly in compliance evaluations and reporting.
- Case Study Insight: A recent case study titled 'Balancing Technology and Patient Experience' underscores the need for a balanced approach in research, where technology is harnessed to enhance patient experience without compromising the human element. A hybrid strategy that merges technology with personal interaction is vital for improving patient experiences and medical outcomes in research studies. Bioaccess® exemplifies this balance by providing personalized support throughout the design process of clinical trials for medical devices in Argentina.
- Current Innovations: Through its technologies, Bioaccess® aims to accelerate the research process and render clinical evaluations more inclusive and accessible, which is essential for designing clinical trials for medical devices in Argentina, mirroring current trends in the integration of technology in clinical research. With an emphasis on navigating the regulatory landscape, including compliance with INVIMA, Bioaccess® ensures that all studies meet the highest standards of safety and efficacy.
By thoughtfully integrating these technologies, researchers can significantly enhance study efficiency and improve the overall participant experience, ultimately expediting the advancement of medical devices in the market.
Post-Trial Considerations: Data Analysis and Reporting
Upon completing a medical study, several vital actions must be taken to ensure the integrity and applicability of the results, particularly when leveraging the comprehensive study management services provided by bioaccess® in Latin America.
- Data Cleaning: It is imperative to verify that all collected data is accurate and complete prior to analysis. This process significantly reduces mistakes and biases that can influence results. By pre-specifying characteristics for subgroup analyses in the protocol, researchers can further mitigate spurious findings and biases in interpretation.
- Statistical Analysis: Employing appropriate statistical methods to analyze the data is crucial, with a focus on assessing both the safety and efficacy of the medical device. Utilizing advanced techniques, such as hierarchical regression models, can enhance understanding of treatment effects and individual differences. For instance, in research involving astronauts, there is a 25 percent likelihood that an astronaut will experience bone mineral density loss during the second year of a 2-year space mission, underscoring the importance of comprehending individual variations in treatment effects.
- Reporting Results: A comprehensive report should be prepared, detailing the methodology, results, and conclusions. This document serves not only as a record of the proceedings but also as an essential resource for regulatory submissions. As highlighted by the Institute of Medicine, the adaptability of decision analysis can assist in establishing priorities for medical investigation and promote the efficient transfer of research findings to practice.
- Regulatory Submission: Findings must be submitted to relevant regulatory bodies, such as INVIMA, ensuring compliance with local regulations. As a Level 4 health authority recognized by PAHO/WHO, INVIMA plays a crucial role in overseeing medical devices in Colombia. Successful regulatory submissions are essential for advancing medical devices from testing phases to market availability.
- Post-Market Surveillance: A robust plan for ongoing monitoring of the device's performance in the market must be developed and implemented. This step is essential for identifying any long-term safety concerns and ensuring ongoing efficacy.
These actions are vital for confirming the results of the study and ensuring the medical device's safety and effectiveness in practical applications, especially when designing clinical trials for medical devices in Argentina. Adhering to best practices in post-trial data analysis and reporting not only enhances the credibility of the findings but also facilitates informed decision-making in research. The utilization of meta-analysis across various fields, particularly in evidence-based medicine, underscores the relevance of robust statistical methods in designing clinical trials for medical devices in Argentina, further supported by the expertise of bioaccess®.
Conclusion
The commitment to advancing clinical trials for medical devices in Latin America is pivotal, enhancing patient outcomes and contributing to local economic growth and international collaboration. By prioritizing strategic partnerships, ethical considerations, and innovative technologies, stakeholders can cultivate a robust ecosystem that supports the successful introduction of groundbreaking medical devices. This ongoing evolution in the clinical research landscape holds significant promise for the future of healthcare in the region, underscoring the necessity of diligence and adaptability in these endeavors.
Frequently Asked Questions
What are the main phases of clinical studies for medical devices?
The main phases include preclinical evaluations, pilot experiments, pivotal assessments, and post-market reviews.
What is the purpose of feasibility assessments in clinical studies?
Feasibility assessments evaluate the viability of conducting a test, including patient recruitment and site selection, which is crucial due to geographical challenges in Latin America.
What are pilot trials and their significance?
Pilot trials are smaller-scale experiments focused on initial safety and effectiveness, allowing researchers to refine protocols before larger studies.
What do pivotal trials aim to achieve?
Pivotal trials provide definitive evidence of a device's effectiveness and safety and are essential for regulatory approval.
What are post-market clinical follow-up assessments (PMCF)?
PMCF assessments track long-term safety and effectiveness of devices after they are available in the market, ensuring continuous regulatory compliance.
Why is efficient patient selection and recruitment important in clinical trials?
Efficient patient selection and recruitment are critical to enhance testing efficiency, reduce costs, and improve success rates, especially in regions with logistical challenges.
What regulatory body oversees clinical trials for medical devices in Argentina?
ANMAT (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica) oversees the approval process for clinical trials in Argentina.
What is required for the design of clinical trials according to ANMAT?
Researchers must submit a comprehensive clinical study dossier, including the study protocol, informed consent forms, and detailed safety data, and obtain ethical committee approval.
What are the risk classifications for medical devices, and why are they important?
Medical devices are categorized into Class I, II, and III based on risk levels, influencing the specific regulatory requirements applicable to each study.
How does bioaccess® support clinical trials for medical devices in Latin America?
Bioaccess® provides services such as feasibility studies, site selection, compliance reviews, project management, and reporting on study status and adverse events to enhance medical device evaluations.




