Overview
This article examines the design of effective trials for wearable technology in Mexico, highlighting the critical aspects of regulatory compliance, participant engagement, and data management. It presents best practices such as:
- Collaborating with local regulatory bodies
- Prioritizing user-friendly technology
- Fostering partnerships with healthcare providers
These strategies collectively enhance the integrity and success of clinical research involving wearables, underscoring the importance of collaboration in navigating the challenges within the Medtech landscape.
Introduction
The emergence of wearable technology is revolutionizing the landscape of clinical trials, presenting unprecedented opportunities for data collection and participant monitoring. Devices such as smartwatches and fitness trackers not only enhance the accuracy of health metrics but also enable researchers to observe patient behaviors in real-world settings. In regions like Latin America, where regulatory frameworks are still evolving, the integration of wearables introduces unique challenges alongside immense potential.
Organizations like bioaccess® are pioneering innovative strategies in this field, making it essential for researchers to understand the implications of wearable technology in clinical trials. This understanding is crucial for improving health outcomes and streamlining the path to regulatory approval.
This article delves into the multifaceted role of wearable technology in clinical research, exploring:
- Regulatory considerations
- Participant recruitment strategies
- Best practices for trial design
while highlighting the transformative impact of these devices on the future of healthcare.
The Role of Wearable Technology in Clinical Trials
Wearable technology has fundamentally transformed the landscape of clinical trials by facilitating continuous information collection and real-time observation of participants. Devices such as smartwatches, fitness trackers, and specialized medical gadgets capture a wide range of health-related metrics, including heart rate, activity levels, and sleep patterns. This comprehensive data collection not only enhances the accuracy of health outcomes but also allows researchers to observe patient behaviors in their natural environments, thereby improving the ecological validity of the findings.
In Latin America, where regulatory frameworks are still evolving, the design of trials for wearables in Mexico holds particular significance for research studies. bioaccess® leverages over 20 years of expertise in managing various studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
to ensure these evaluations are conducted efficiently and in compliance with local regulations, including oversight by INVIMA, Colombia's National Food and Drug Surveillance Institute. bioaccess® adopts a customized approach to trial management, tailoring services to meet the unique needs of each study.
Recent statistics reveal that 73% of studies utilizing body-worn technology employed commercially available devices that are not currently regulated by the US Food and Drug Administration or the Therapeutic Goods Administration in Australia. This highlights the growing reliance on devices in medical research, particularly in regions like Latin America, where the design of trials for wearables in Mexico positions bioaccess® as a leader in innovation and regulatory excellence.
The integration of devices into clinical trials has demonstrated substantial advantages. For instance, a synthesis of 155 outcome measures from various studies, including the case study titled "Mapping Outcomes to the Quadruple Aim of Health Care," indicated that 89.7% of these measures focused on healthcare outcomes, underscoring improvements in pain management, quality of life, and physical function. Notably, while the impact of devices on patient experience was thoroughly examined, the cost-effectiveness of device-guided care was also highlighted, suggesting it could be more economical than conventional methods.
Moreover, devices facilitate real-time information gathering, reducing the necessity for regular face-to-face appointments and providing clearer insights into patients' daily routines and health status. This capability is particularly advantageous in the context of medical studies, where prompt and precise information is critical for evaluating treatment effectiveness. As noted by Maddison et al., the primary outcome measure studied was maximal oxygen consumption (VO max), which refers to the maximum amount of oxygen an individual can utilize during maximal exercise and serves as a marker of cardiovascular fitness.
In conclusion, the adoption of portable technology in research studies not only enhances information integrity and validation but also accelerates the regulatory approval process. Retaining raw sensor data is essential to meet regulatory requirements concerning data integrity, verification, and validation, making these devices invaluable assets for researchers in the Medtech sector. With bioaccess® at the forefront of medical research in Latin America, the impact of these studies extends beyond individual experiments, contributing to job creation, economic development, and the enhancement of healthcare in the region.
Understanding Regulatory Frameworks for Wearable Trials in Mexico
In Mexico, the regulatory framework governing research involving smart devices is primarily shaped by the Federal Law of Health and the specific guidelines established by COFEPRIS (Federal Commission for the Protection against Sanitary Risk). A critical first step for researchers is the accurate classification of wearable devices, as certain devices may not require registration as medical devices. This classification is essential for effectively navigating the regulatory requirements.
Adherence to the NOM-241-SSA1-2021 standards is paramount, as these guidelines delineate the good manufacturing practices required for medical devices.
Moreover, acquiring informed consent from participants regarding data gathering and privacy is not merely a best practice; it is a legal obligation that safeguards participant rights and data integrity.
At bioaccess®, we acknowledge the challenges faced by medical device startups in research studies, including regulatory hurdles, competition, recruitment difficulties, and financial constraints. Our comprehensive services encompass:
- Feasibility studies
- Selection of research locations and lead investigators
- Study set-up
- Start-up approvals
- Reporting on study status, inventory, and adverse occurrences
This thorough evaluation is essential for adherence and success in experiments.
Expert insights underscore the importance of understanding COFEPRIS regulations, particularly in the context of designing trials for wearables in Mexico. Additionally, it is crucial to recognize that the maximum response time for a UHAP evaluation request is 30 calendar days, providing a clearer timeline for the regulatory process. Case studies illustrate the necessity for sponsors to adhere to Good Clinical Practice guidelines, emphasizing the importance of clearly defining roles and responsibilities when engaging a Contract Research Organization (CRO).
By establishing these explicit guidelines, the quality and integrity of research findings are enhanced, ensuring that all parties involved comprehend their responsibilities and the regulatory landscape. Furthermore, since Mexico is a signatory to the Nagoya Protocol, researchers conducting studies that involve non-human genetic resources must be cognizant of the implications this may have on their work. At bioaccess®, we are dedicated to ensuring information security and client trust, addressing any concerns through our grievance and data protection procedures.
If you have any questions regarding the handling of your information, please feel free to contact our Grievance Officer at info@bioaccessla.com.
In summary, a thorough understanding of COFEPRIS guidelines and the regulatory requirements for technology studies is essential for designing trials for wearables in Mexico, ensuring the successful execution of clinical research. This knowledge not only facilitates compliance but also accelerates the pathway for innovative medical devices to reach the market. BOOK A MEETING.
Strategies for Participant Recruitment in Wearable Trials
Designing trials for wearables in Mexico necessitates a thorough and strategic approach for participant recruitment in these studies. It begins with a clear definition of the target population, considering their demographics, health conditions, and technological familiarity. Engaging the community is crucial; forging collaborations with local healthcare providers and patient advocacy organizations fosters trust and heightens awareness about the study.
Significantly, bioaccess™ has partnered with Caribbean Health Group to position Barranquilla as a premier site for clinical research in Latin America, a commitment supported by Colombia's Minister of Health. This partnership, announced on March 29, 2019, enhances the reliability of experiments and encourages local involvement.
Efficiently leveraging social media and digital platforms can broaden outreach, allowing prospective participants to understand the benefits of joining the study, including access to cutting-edge technology and enhanced health monitoring. Incorporating incentives can substantially boost recruitment efforts. Offering health assessments or financial compensation not only attracts participants but also emphasizes the value of their contributions to advancing medical technology.
Statistics reveal that diverse recruitment strategies improve the evaluation of safety and efficacy across varied populations, making it vital to adopt a multifaceted approach. A case study utilizing unsupervised K-means clustering analysis highlighted distinct engagement patterns among participants, underscoring the necessity of tailoring recruitment strategies to different data streams. This insight can guide the development of focused engagement strategies that resonate with specific demographic groups, ultimately yielding more effective recruitment outcomes in health studies involving trials for wearables in Mexico. Furthermore, GlobalCare Clinical Trials' partnership with bioaccess™ has resulted in over a 50% reduction in recruitment time and an impressive 95% retention rate, illustrating the effectiveness of strategic collaborations in clinical research.
As Matthew Hotopf, principal investigator for the RADAR-MDD study, emphasized, understanding participant engagement is essential for optimizing recruitment strategies. Recent studies have also indicated high satisfaction with the virtual informed consent process, reinforcing the significance of participant experience in recruitment efforts.
Key Design Elements for Effective Wearable Trials
Designing trials for wearables in Mexico is crucial for advancing clinical research. The selection of appropriate wearable devices is paramount, necessitating validation for both accuracy and reliability. This validation is essential, as it directly impacts the quality of data gathered and the overall integrity of the results.
The potential of wearable technologies to enhance personalized medicine through improved detection of treatment effects underscores the importance of these trials. Adaptive study designs represent a significant evolution in clinical research methodologies, allowing for adjustments based on interim outcomes. This flexibility not only enhances the responsiveness of the experiment but also optimizes resource allocation and participant engagement.
For example, studies indicate that adaptive designs can lead to more efficient trials, significantly reducing the time and costs associated with traditional fixed designs.
Integrating digital health technologies, such as mobile applications for information collection and participant engagement, is another critical component. These tools streamline processes, facilitate real-time information capture, and enhance the overall quality of the information gathered. Continuous data collection via devices can greatly improve understanding of treatment reactions, ultimately leading to better patient outcomes.
Establishing clear protocols for information management and analysis is essential to ensure that the collected data is both actionable and relevant. This involves setting standards for data selection and assessment, which can guide the choice of wearable devices and the overall study design. Recent statistics suggest that organized criteria in device selection can lead to more effective assessments, highlighting the importance of meticulous planning during the design phase.
Moreover, case studies reveal the challenges faced by R&D and healthcare institutions in conducting trials for wearables in Mexico. The case study titled 'Challenges in Implementing Wearable Technology' illustrates specific obstacles, such as the need for analytical and medical validation techniques and ensuring device acceptability among participants. Addressing these challenges through stakeholder collaboration and refining validation processes is vital for the successful integration of devices in clinical research.
As John Wagner noted, healthcare services or products must be clinically suitable and not primarily profit-driven, a consideration especially relevant when evaluating the standards of care in studies involving wearable devices.
By focusing on these essential components, researchers can design studies that not only meet regulatory requirements but also advance the field of personalized medicine. bioaccess® plays a pivotal role in connecting innovative Medtech companies with research opportunities in Latin America. With over 20 years of experience, bioaccess® offers comprehensive management services for studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting.
This expertise facilitates the successful execution of trials for personal devices, ensuring they are conducted efficiently and effectively within the Latin American market.
Data Management and Analysis in Wearable Clinical Trials
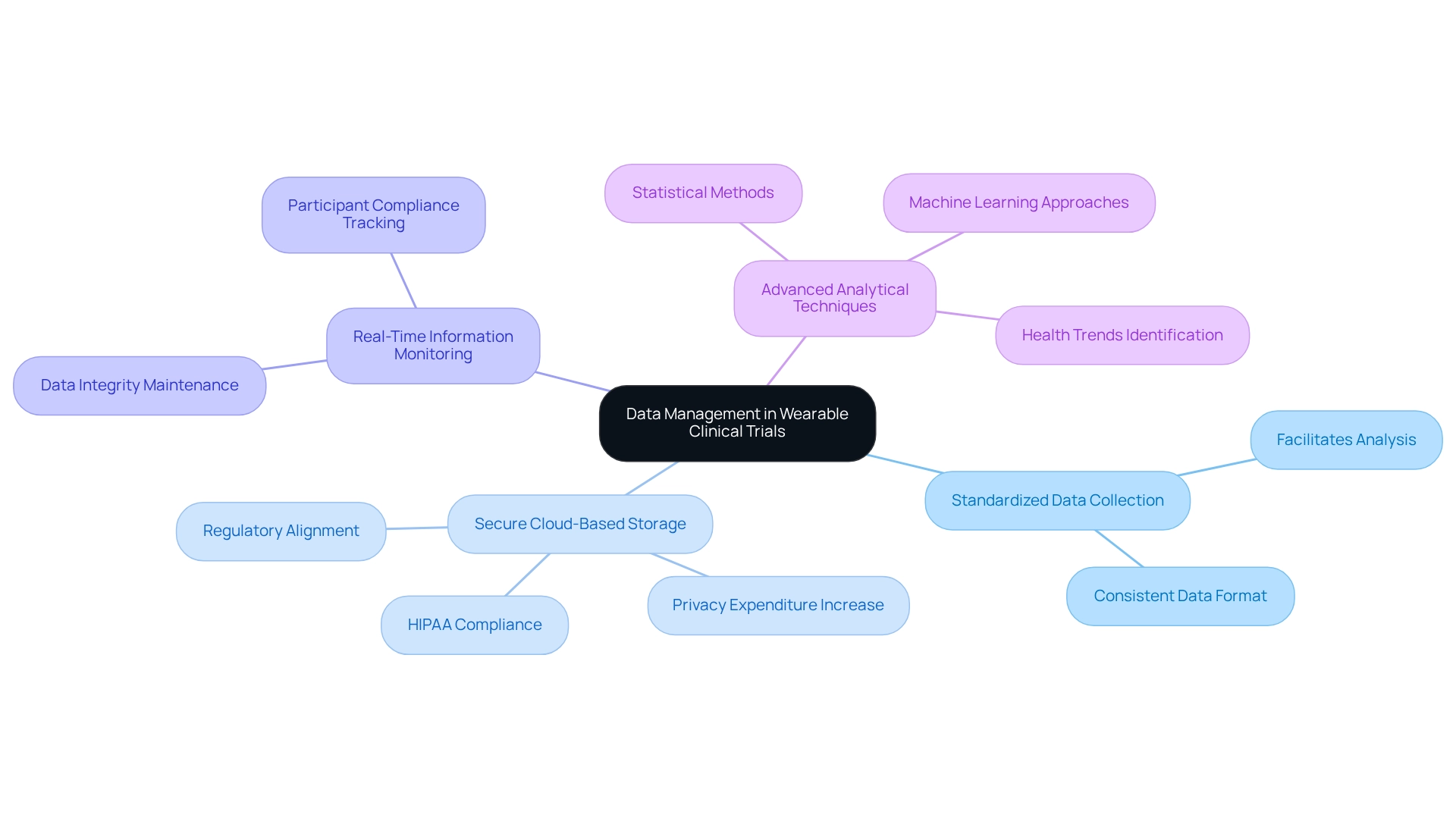
Efficient information management is paramount in designing trials for wearables in Mexico, as it safeguards the integrity and security of participant details. Key practices include:
- Standardized Data Collection: Collecting data in a consistent format is essential for facilitating analysis and ensuring comparability across different study sites.
- Secure Cloud-Based Storage: Utilizing secure cloud-based platforms not only protects participant information but also aligns with increasing regulatory demands. With anticipated expenditure on privacy compliance expected to rise by 15-20% each year until 2025, investing in robust storage solutions is more critical than ever. Adhering to HIPAA and state regulations regarding the collection, storage, and sharing of wearable information is vital to uphold participant trust and information integrity.
- Real-Time Information Monitoring: Implementing systems for real-time monitoring enables researchers to track participant compliance and maintain information integrity throughout the trial. This proactive strategy can significantly enhance the reliability of the collected data.
- Advanced Analytical Techniques: Employing advanced statistical methods and machine learning approaches allows researchers to extract deeper insights from the information. These methodologies can uncover significant health trends and treatment effects, ultimately leading to more informed decision-making.
A notable case study illustrates how advanced information management practices have revolutionized drug development. By adopting comprehensive information management solutions, pharmaceutical companies reduced their drug development cycle by an average of 1.5 years and improved their chances of regulatory approval by 23%. This emphasizes the potential benefits of efficient information management, not only in drug development but also in the context of designing trials for wearables in Mexico.
As bioaccess® emphasizes, "bioaccess® is committed to ensuring information security and client trust, with a dedicated Grievance Officer available to address any concerns regarding information processing, thus ensuring compliance and transparency in all operations." This dedication to information security is crucial as the landscape of clinical research evolves. Staying abreast of contemporary developments in information analysis and management techniques is essential for success in designing trials for wearables in Mexico.
Furthermore, bioaccess® offers extensive services such as feasibility assessments, site selection, and regulatory compliance support to assist medical device startups in managing testing efficiently.
Benefits and Challenges of Wearable Technology in Trials
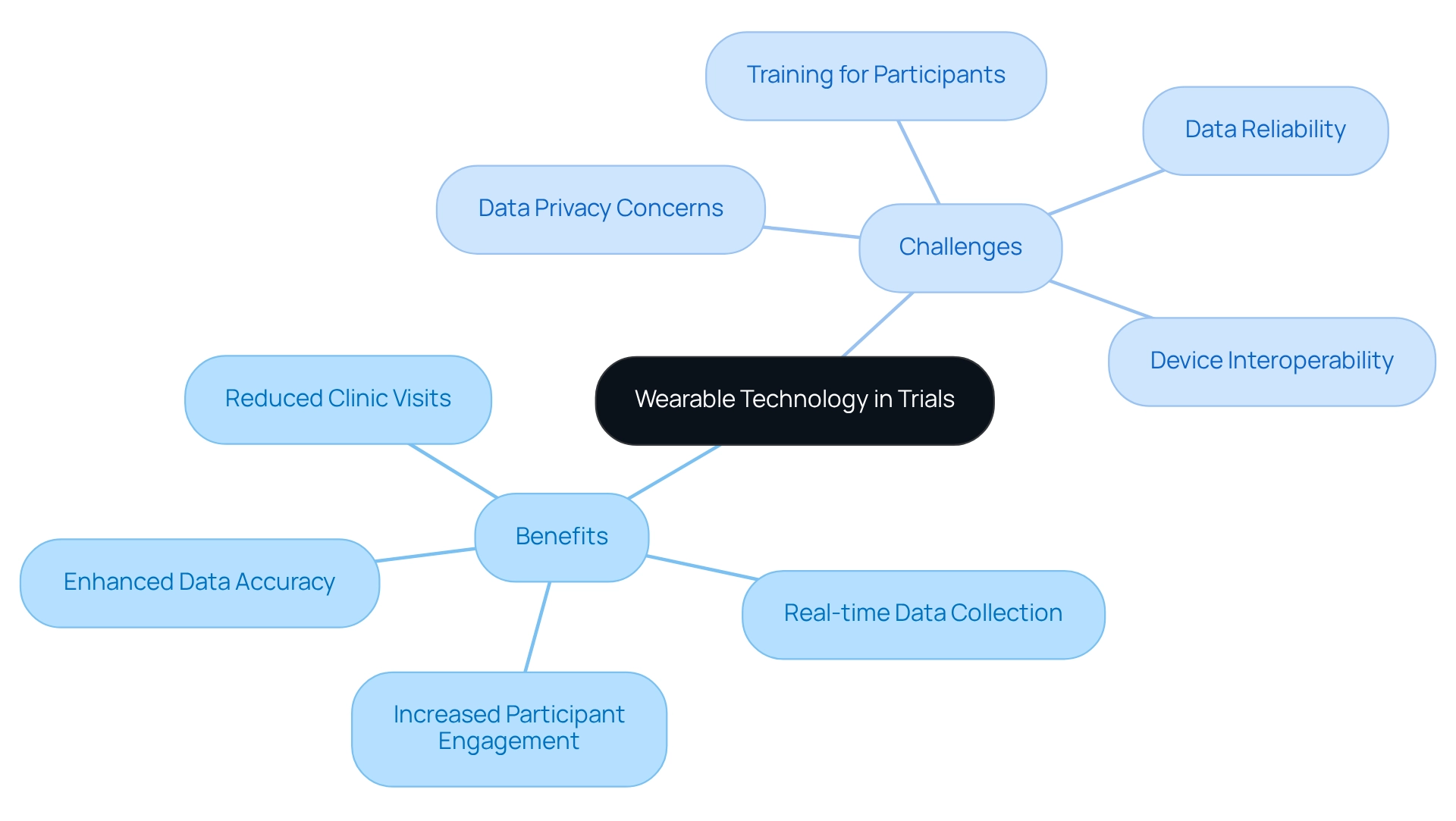
Designing trials for wearables in Mexico offers a transformative opportunity in medical research, delivering significant benefits such as enhanced data accuracy, increased participant engagement, and the capacity to collect data in real-time. These devices can reduce the need for frequent clinic visits, alleviating participant burden and boosting retention rates. For instance, studies indicate that devices supported the monitoring of diagnosed conditions or at-risk patients in 4.5% of clinical research, underscoring their potential utility across various settings.
However, several challenges must be addressed to fully harness this technology in clinical research. The notable lack of research on devices in low-resource environments highlights the necessity of designing trials for wearables in Mexico, often stemming from insufficient funding and skepticism regarding their efficacy. Additionally, data privacy concerns remain critical, as participants may feel uneasy about how their information is utilized and safeguarded.
Moreover, device interoperability can pose significant challenges, as differing technologies may not seamlessly integrate, complicating data collection and analysis. Furthermore, adequate training for participants is essential in designing trials for wearables in Mexico to ensure effective device usage, which can impact data quality and participant adherence.
The reliability of data collected from devices can also fluctuate, necessitating a meticulous approach to device selection and validation. As Xu et al. noted, physiological monitoring with devices offers promise for considerable improvements in neonatal outcomes in low- and middle-resource countries.
Maintaining raw sensor data is crucial, as it serves as the primary source for deriving digital medical measures, ensuring compliance with regulatory standards regarding data integrity and verification. By addressing these challenges through thoughtful planning and execution, researchers can unlock the full potential of portable technology, ultimately leading to more effective and efficient clinical studies.
Best Practices for Designing Wearable Trials in Mexico
Designing effective wearable trials in Mexico necessitates a strategic approach that incorporates several best practices:
-
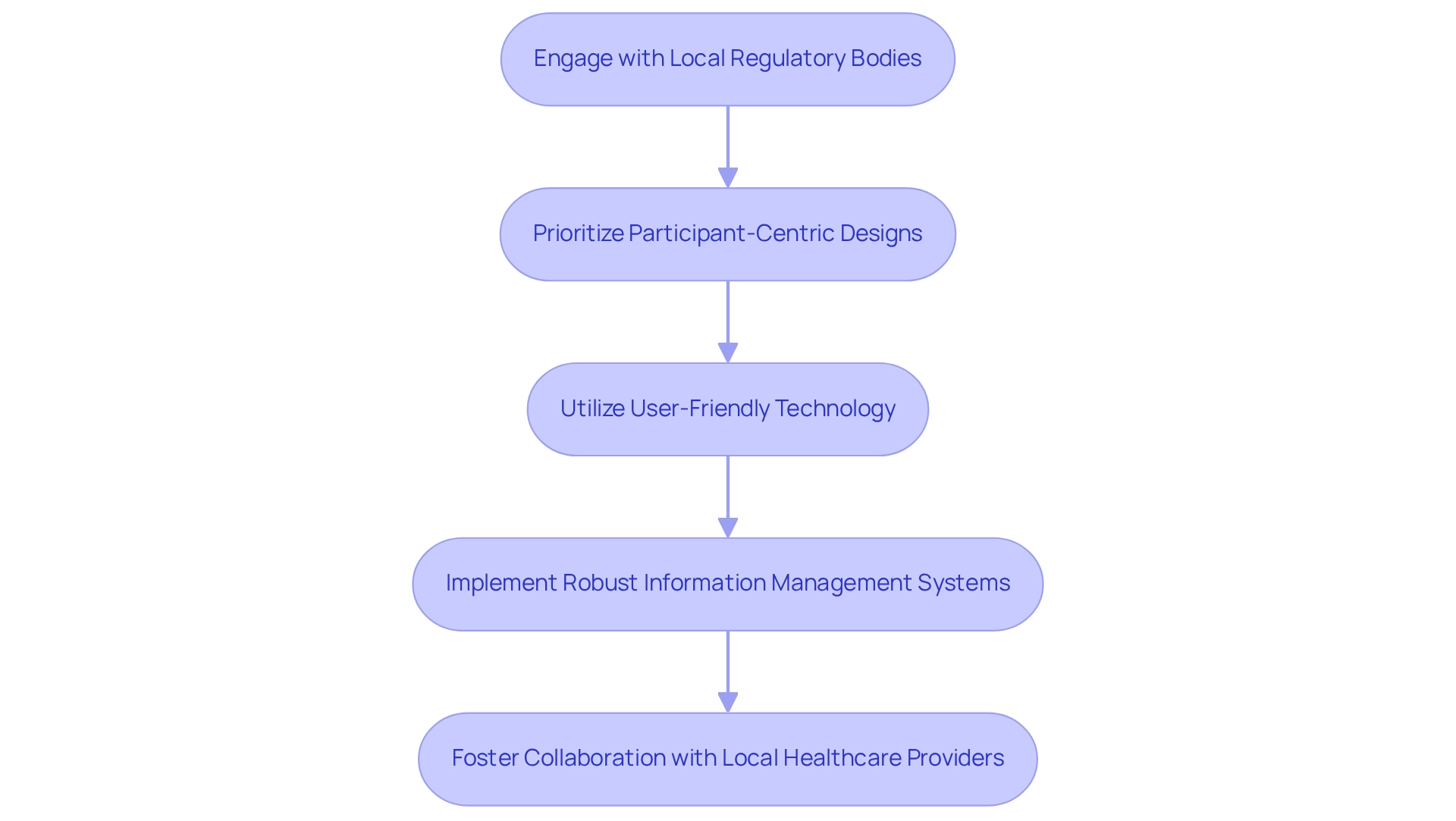
Engage with Local Regulatory Bodies: Initiating discussions with local regulatory authorities early in the planning phase is crucial. This proactive engagement ensures compliance with legal requirements and facilitates smoother approval processes. Given that research in body-worn technology must achieve a compliance rate exceeding 80% for accuracy validation, addressing regulatory adherence is vital to the success of the experiments. In this context, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential as it oversees medical device regulations and classifications, acting as a Level 4 health authority by PAHO/WHO.
-
Prioritize Participant-Centric Designs: Understanding the cultural and social contexts of the target population is imperative. Trials should be designed with the participants' needs in mind, ensuring that the wearable devices are not only effective but also resonate with the users' lifestyles and preferences.
-
Utilize User-Friendly Technology: The technology employed in trials should be accessible and easy to use. Enhancing participant involvement through user-friendly interfaces can significantly improve information collection and compliance with study protocols.
-
Implement Robust Information Management Systems: Ensuring integrity and security is paramount. Researchers should implement thorough information management systems that protect participant details while enabling effective analysis. As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We conducted a small AI initiative to determine if we can produce significant test information for configuring and validating our systems. It turns out we can." The integration of AI-driven analytics, as highlighted in recent case studies, can revolutionize data handling and patient monitoring, leading to improved health outcomes and enhanced patient engagement. This innovation aligns with bioaccess's commitment to utilizing advanced technologies in clinical study management.
-
Foster Collaboration with Local Healthcare Providers: Building strong partnerships with local healthcare institutions can enhance recruitment and retention efforts. Collaborations can provide valuable insights into the local healthcare environment, improving the overall implementation of studies.
By adhering to these best practices, researchers can enhance their efforts in designing trials for wearables in Mexico, ultimately advancing medical technology and improving patient outcomes. The anticipated impact of AI-driven analytics on patient engagement and compliance, as explored in the case study titled "Future Trends in Wearable Technology for Clinical Trials," underscores the significance of these innovations in achieving successful clinical research. Furthermore, leveraging the expertise of bioaccess® in managing various study types—including Early-Feasibility and First-In-Human studies—alongside comprehensive services like feasibility studies, site selection, trial setup, and project management, can substantially enhance the trial process.
Conclusion
The integration of wearable technology into clinical trials is revolutionizing research, offering significant advantages such as real-time data collection, enhanced accuracy, and improved participant engagement. Devices like smartwatches and fitness trackers enable researchers to capture health metrics in real-world environments, thereby boosting the ecological validity of their findings. In regions such as Latin America, organizations like bioaccess® are skillfully navigating evolving regulatory frameworks to ensure compliance and foster innovation.
A comprehensive understanding of regulatory guidelines, particularly those from COFEPRIS in Mexico, is crucial for successful trial execution. This encompasses accurate device classification, obtaining informed consent, and adhering to local regulations to protect participant rights and streamline approvals. Collaborating with local healthcare providers can further enhance recruitment and retention, ultimately leading to more effective trials.
Effective trial design is paramount for maximizing the benefits of wearables. By selecting user-friendly devices, implementing robust data management systems, and employing adaptive trial methodologies, researchers can optimize data collection and analysis. These strategies not only ensure compliance but also advance personalized medicine approaches.
In summary, wearable technology presents both challenges and opportunities within clinical trials. By embracing best practices and fostering collaboration among stakeholders, researchers can significantly enhance the efficiency and effectiveness of clinical research, thereby driving better health outcomes and innovations in medical technology.
Frequently Asked Questions
How has wearable technology impacted clinical trials?
Wearable technology has transformed clinical trials by enabling continuous information collection and real-time observation of participants, enhancing the accuracy of health outcomes and allowing researchers to observe patient behaviors in their natural environments.
What types of health-related metrics can wearable devices capture?
Wearable devices can capture a variety of health-related metrics, including heart rate, activity levels, and sleep patterns.
What is the significance of wearable trials in Mexico?
In Mexico, the design of trials for wearables is significant due to evolving regulatory frameworks, and bioaccess® utilizes its expertise to conduct studies efficiently while complying with local regulations.
What types of studies does bioaccess® manage?
bioaccess® manages several types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What percentage of studies using body-worn technology utilize unregulated devices?
Recent statistics show that 73% of studies utilizing body-worn technology employed commercially available devices that are not currently regulated by the US Food and Drug Administration or the Therapeutic Goods Administration in Australia.
What advantages do wearable devices offer in clinical trials?
Wearable devices provide substantial advantages such as real-time information gathering, reducing the need for regular face-to-face appointments, and offering clearer insights into patients' daily routines and health status.
What is the primary outcome measure studied in relation to wearable devices?
The primary outcome measure studied is maximal oxygen consumption (VO max), which indicates the maximum amount of oxygen an individual can utilize during maximal exercise, serving as a marker of cardiovascular fitness.
What regulatory framework governs research involving smart devices in Mexico?
The regulatory framework is shaped by the Federal Law of Health and specific guidelines established by COFEPRIS (Federal Commission for the Protection against Sanitary Risk).
Why is the classification of wearable devices important?
Accurate classification of wearable devices is essential because certain devices may not require registration as medical devices, which helps navigate regulatory requirements effectively.
What are the key responsibilities of researchers regarding informed consent?
Researchers must acquire informed consent from participants regarding data gathering and privacy, as it is a legal obligation that safeguards participant rights and data integrity.
What challenges do medical device startups face in research studies?
Medical device startups encounter challenges such as regulatory hurdles, competition, recruitment difficulties, and financial constraints.
What is the maximum response time for a UHAP evaluation request in Mexico?
The maximum response time for a UHAP evaluation request is 30 calendar days.
What guidelines must sponsors adhere to when conducting studies?
Sponsors must adhere to Good Clinical Practice guidelines, which emphasize the importance of clearly defining roles and responsibilities when working with a Contract Research Organization (CRO).
How does bioaccess® ensure information security and client trust?
bioaccess® ensures information security and client trust by addressing concerns through grievance and data protection procedures.




