Overview
To ensure compliance for clinical trials under COFEPRIS, researchers must adhere to a structured, step-by-step process. This process includes:
- Obtaining necessary approvals from ethics committees and the regulatory authority
- Maintaining meticulous documentation and ethical oversight
The importance of strategic planning and adherence to guidelines cannot be overstated. Organizations like bioaccess® play a critical role in navigating these complexities, facilitating successful study outcomes. Their expertise is essential for researchers aiming to overcome the challenges inherent in clinical trials.
Introduction
In the rapidly evolving landscape of clinical research, understanding the regulatory framework is paramount for success, particularly in Mexico, where COFEPRIS, the Federal Commission for Protection against Sanitary Risks, plays a crucial role. This authoritative body oversees clinical trials and ensures the safety and efficacy of medical products, making compliance with its guidelines essential for researchers and sponsors alike.
As the demand for innovative medical solutions grows, navigating the intricacies of COFEPRIS regulations becomes increasingly vital. From the approval process to ethical considerations and post-market follow-up, this article delves into the comprehensive steps necessary to conduct compliant clinical trials in Mexico.
It highlights the pivotal role of strategic planning and meticulous execution in advancing medical technology and improving patient outcomes.
Understanding COFEPRIS: The Regulatory Authority for Clinical Trials in Mexico
The Federal Commission for Protection against Sanitary Risks serves as Mexico's central regulatory authority, overseeing research involving human participants. Established to safeguard the security and effectiveness of medical products, this agency operates under the Ministry of Health, playing a crucial role in advancing medical research. Understanding the agency's regulations is vital for researchers and sponsors, as compliance for clinical trials under COFEPRIS encompasses the approval procedure for trials, the oversight of ongoing studies, and the enforcement of adherence to both national and international standards.
Recent updates to the regulatory guidelines underscore the need for stringent ethical oversight, particularly in studies involving vulnerable populations, such as minors. Researchers must ensure that the risks associated with such studies are justified by the potential benefits, with strict supervision mandated to evaluate any risks that may affect participants' welfare.
Statistically, sponsors are obligated to retain essential documentation for a minimum of five years following product registration, or for at least two years after the last marketing approval or formal discontinuation of clinical development. This requirement highlights the importance of meticulous record-keeping in ensuring compliance for clinical trials under COFEPRIS.
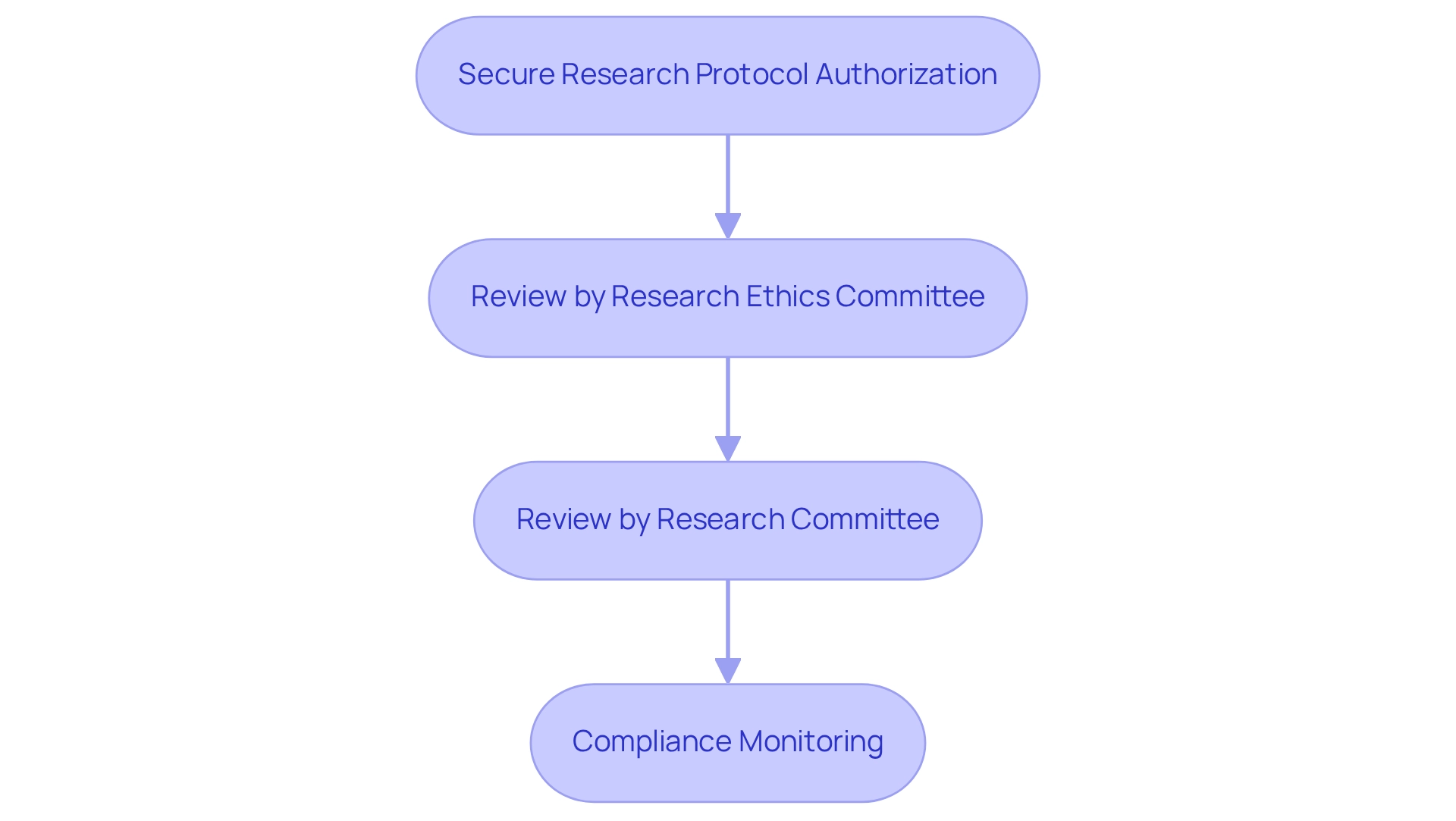
A critical aspect of the approval process is the requirement for applicants to secure research protocol authorization from the regulatory body, ensuring compliance for clinical trials under COFEPRIS, alongside favorable decisions from both the Research Ethics Committee and the Research Committee at the health institution conducting the study. This sequential review process, which does not allow for simultaneous assessments, can extend the timeline for obtaining essential approvals, emphasizing the need for strategic planning in research timelines. For instance, the Research Protocol Authorization Process of the agency illustrates how the absence of simultaneous reviews can lead to delays, necessitating thorough planning by researchers.
As a leading contract research organization (CRO) in Latin America, bioaccess® is dedicated to providing comprehensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. With over 20 years of expertise in Medtech, bioaccess® is well-equipped to navigate the complexities of regulatory standards, ensuring that your trials are executed effectively and in accordance with all required guidelines.
bioaccess® prioritizes data protection and compliance, guaranteeing that all client information is managed with the utmost care and in line with applicable laws. Specialist perspectives on the agency's role in medical studies highlight its significance in ensuring compliance for clinical trials under COFEPRIS and fostering an ethical research environment. As articulated by Martin Borrego Llorente, Chief of Staff to Foreign Secretary Alicia Barcena, "There are many components to the progress that has been made, but the real impact to the user is extraordinary…It is much more than a platform; it changes the dynamic between the user and the public servant."
This viewpoint accentuates the advancements achieved in regulatory procedures and their transformative influence on the relationship between researchers and regulatory agencies, enhancing the overall efficacy of medical studies in Mexico. Comprehending these guidelines and the regulatory landscape is essential for ensuring compliance for clinical trials under COFEPRIS and propelling medical innovations.
Step-by-Step Process for Obtaining Clinical Trial Approval from COFEPRIS
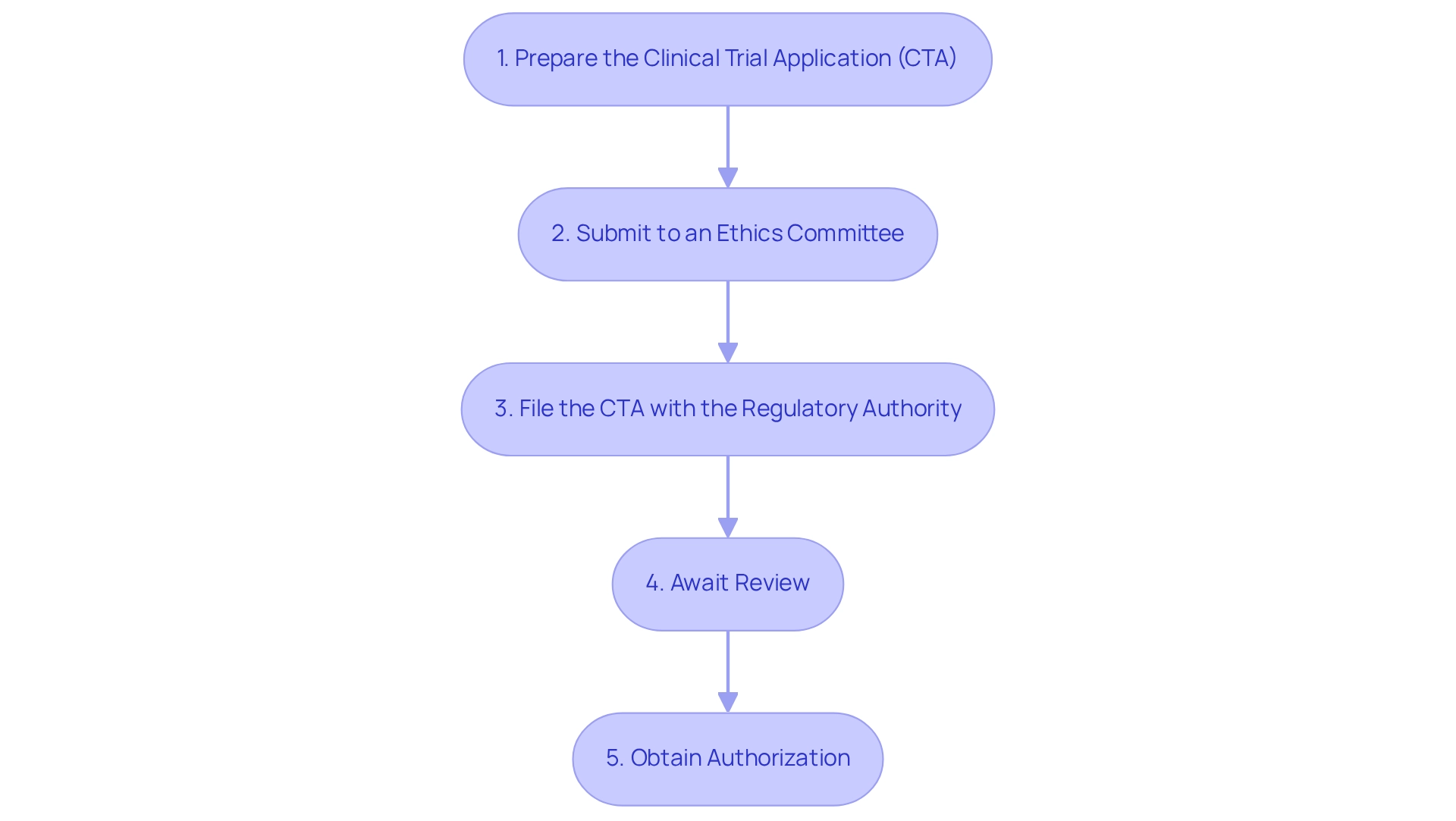
To successfully secure research study approval from COFEPRIS, it is essential to follow these organized steps:
- Prepare the Clinical Trial Application (CTA): Gather all essential documents, including the study protocol, informed consent forms, and qualifications of the investigators. This step is vital for a seamless application procedure and demonstrates bioaccess®'s commitment to supporting medical device clinical research in Latin America.
- Submit to an Ethics Committee: Obtain approval from a registered ethics committee, which will assess the ethical aspects of your study, ensuring that participant rights and safety are prioritized—a core principle in navigating the Latin American Medtech landscape.
- File the CTA with the regulatory authority: After obtaining ethics approval, submit the CTA along with any applicable fees to the designated agency. It is crucial to ensure that all documents are thorough and fully compliant with regulatory guidelines to prevent delays, underscoring the significance of regulatory excellence in research management.
- Await Review: The health authority typically examines applications within a maximum of 30 calendar days. During this period, be prepared to address any inquiries or requests for additional information that may arise, as responsiveness can significantly impact the approval timeline. This step highlights the need for effective project management and communication strategies.
Obtain Authorization: Following a successful evaluation, you will receive a notification from COFEPRIS granting permission to commence your research study. Adhering precisely to the approved protocol during the study is crucial to ensure compliance for clinical trials under COFEPRIS and to guarantee participant safety.
In recent years, the medical research application process has undergone enhancements, particularly in response to challenges highlighted by the COVID-19 pandemic. This has led to a push for harmonization of technical requirements and the adoption of ICH guidelines, aimed at improving the efficiency of regulatory processes in the region. Investigational products, including new chemical entities, generics, and medical devices, are utilized for scientific research purposes and are subject to these regulations.
Moreover, case studies demonstrate that in emergency medical situations, regulations permit the use of investigational drugs without prior consent if the participant's condition necessitates immediate action, provided that the ethics committee is informed afterward. This flexibility underscores the importance of prioritizing participant safety while ensuring compliance for clinical trials under COFEPRIS and maintaining ethical standards. As noted by Ms. Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, the E22 Guideline seeks to enhance the utilization of patient preference information as input for pharmaceutical product development through a globally harmonized framework, emphasizing the significance of integrating patient perspectives in clinical studies.
The Role of Clinical Trials in Medical Device Development and Compliance
Clinical studies play a pivotal role in the development of medical devices, confirming safety and effectiveness prior to market entry. These essential tests yield critical information that informs regulatory submissions, ensuring compliance with clinical trials under COFEPRIS and other regulatory bodies. By executing meticulously designed research studies, companies can demonstrate that their devices function as intended, instilling confidence among healthcare providers and patients alike.
Moreover, successful tests can expedite approval processes and broaden market access, ultimately enhancing patient outcomes.
In 2025, the significance of research studies in medical device development is underscored by their ability to provide vital information on treatment safety, dosage, and pharmacokinetics. These insights are instrumental in guiding crucial decisions regarding the continuation of development and subsequent studies. For instance, Phase 1 studies are particularly vital, as they lay the groundwork for understanding a treatment's safety profile and shaping future research pathways.
The case study titled "Avantec Vascular Chooses bioaccess™ For A First-In-Human Research Study In Latin America" exemplifies how bioaccess aids companies in navigating the complexities of medical studies, including site selection, regulatory dossier submission, and ensuring compliance for clinical trials under COFEPRIS, thereby increasing the likelihood of successful outcomes.
It is also essential for investigators to return any unused stock of the investigational device to the study sponsor if the investigation is terminated, suspended, discontinued, or completed. This requirement is a crucial element of compliance for clinical trials under COFEPRIS, emphasizing the importance of adhering to established protocols.
Recent trends reveal a heightened focus on ethical considerations, scientific rigor, and collaborative partnerships in medical research. These elements are vital for maintaining the integrity and impact of research studies, ensuring compliance for clinical trials under COFEPRIS while also contributing significantly to advancements in medical technology. As Langevin noted, the strength of interconnected spinal stimulation illustrates the innovative possibilities that medical studies can unveil.
As the landscape of research studies evolves, the success rates for medical devices continue to improve, reflecting the increasing complexity of study designs and methodologies.
Furthermore, research studies exert a considerable influence on local economies, contributing to job creation, economic development, and the enhancement of healthcare. Ultimately, the importance of research studies in medical device advancement cannot be overstated. They are not merely a regulatory hurdle but a fundamental aspect of bringing innovative solutions to market, thereby improving healthcare outcomes and elevating the quality of life for patients.
Designing Compliant Clinical Trials: Ethical Considerations and Committee Involvement
Creating compliant research studies necessitates a meticulous focus on ethical considerations. The following key steps are essential:
- Informed Consent: It is crucial that all participants provide informed consent, fully comprehending the associated risks and advantages of the study. Recent statistics indicate that informed consent rates in research studies have improved; however, challenges remain in ensuring that all participants are sufficiently informed.
- Ethics Committee Review: Presenting the study design to an independent ethics committee for evaluation and endorsement is essential. This step ensures adherence to established ethical standards, which are increasingly recognized as vital for maintaining participant trust and integrity in research. As emphasized by John Carreyrou, transparency and ethical integrity are crucial to prevent the pitfalls of oversight failures that can lead to significant problems during research trials.
- Risk-Benefit Analysis: A comprehensive risk-benefit analysis must be conducted to evaluate the potential advantages of the research against the risks posed to participants. This analysis is not only a regulatory requirement but also a moral obligation to safeguard participant welfare. Researchers have a duty to ensure the safety, comfort, and well-being of participants during studies.
- Ongoing Monitoring: Implementing mechanisms for continuous oversight of participant safety throughout the study is essential. This includes provisions for promptly reporting any adverse events, thereby reinforcing the commitment to participant well-being. bioaccess® offers robust project management and monitoring services to ensure that all aspects of participant safety are addressed effectively.
- Transparency: Maintaining transparency with participants about their rights and the study's objectives fosters trust and cooperation. Clear communication is fundamental in ensuring that participants feel valued and informed throughout the research process. bioaccess® is dedicated to guaranteeing information security and client confidence, with established complaint and privacy procedures to address any issues that may arise.
In 2025, ethical considerations in trial design are more crucial than ever, with ongoing discussions emphasizing the necessity for strong oversight mechanisms. The case study on ethical oversight in big data and AI highlights the ethical issues associated with data privacy and algorithmic bias, which are becoming increasingly significant in medical research. Furthermore, the updated curriculum includes 15 expanded modules, emphasizing the importance of ongoing education and training for ethics committees and researchers to ensure compliance with ethical standards.
Moreover, conversations about operational resilience beginning with the supply chain frame the significance of ethical considerations in the wider scope of research and operational integrity. Case studies have demonstrated that effective informed consent procedures greatly improve participant engagement and compliance, ultimately resulting in more dependable research outcomes.
Navigating Challenges in Clinical Trials: Recruitment and Site Selection Strategies
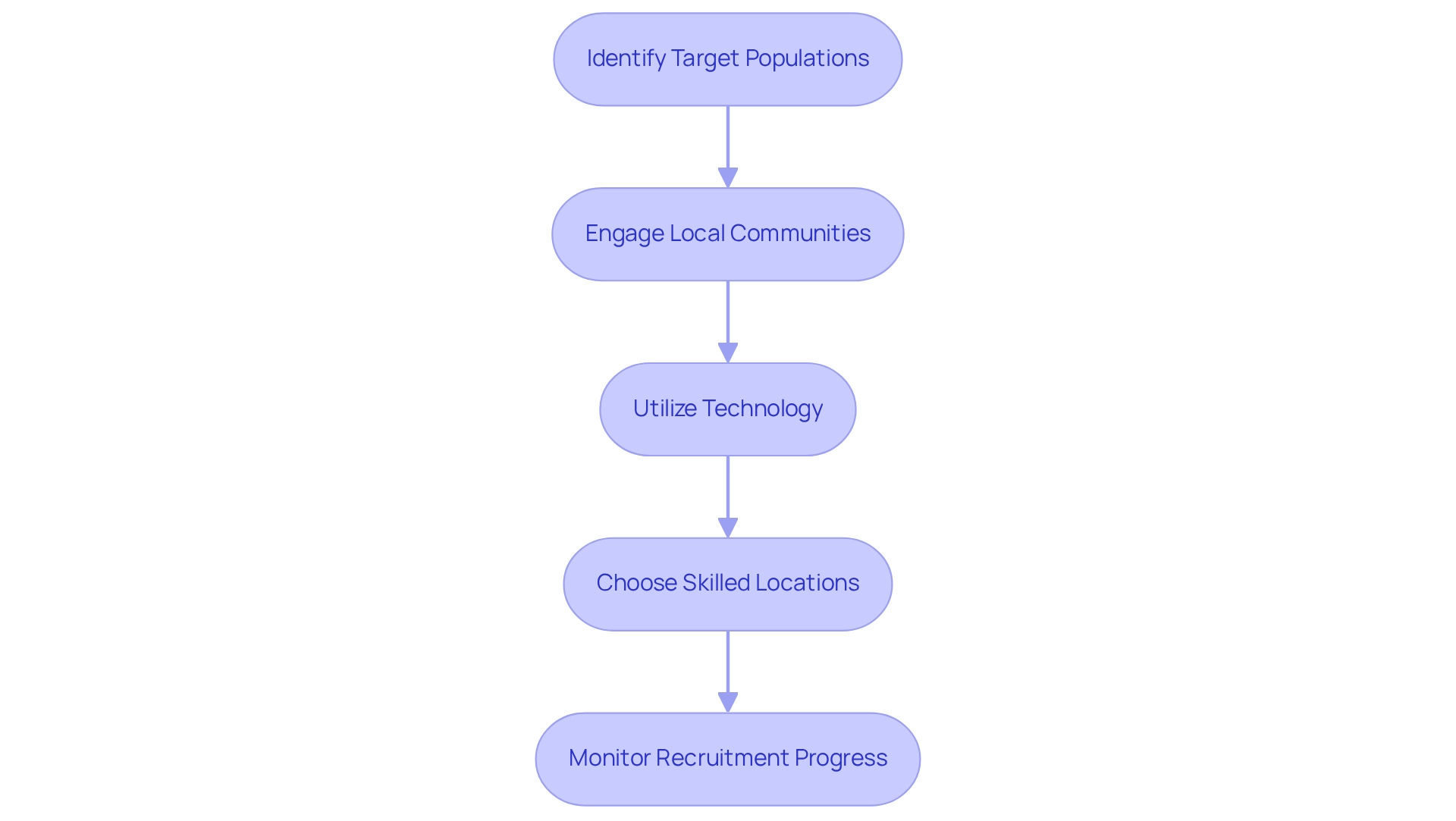
Recruitment and site selection are essential yet frequently challenging aspects of conducting clinical studies. To effectively navigate these hurdles, consider implementing the following strategies:
- Identify Target Populations: Clearly define the target group for your study, ensuring that the inclusion and exclusion criteria are both realistic and achievable. This precision is essential for enhancing recruitment success rates.
- Engage Local Communities: Establish strong relationships with local healthcare providers and community organizations. This engagement not only increases awareness about the study but also builds trust and interest among potential participants, which is crucial for recruitment.
- Utilize Technology: Harness the power of digital platforms and social media to broaden your reach. These tools can streamline the recruitment process, making it easier to connect with diverse populations and enhance participation rates.
- Choose Skilled Locations: Opt for research sites that have a demonstrated history of conducting studies. Ensure these sites possess the necessary infrastructure and trained staff to facilitate smooth operations and effective participant management. For instance, bioaccess™ highlights the practicality and choice of research locations and lead investigators (PIs) as part of their extensive management services for studies, which includes ensuring compliance for clinical trials under COFEPRIS during evaluations and setup procedures.
- Monitor Recruitment Progress: Regularly evaluate recruitment metrics to gauge progress. Be prepared to adapt your strategies based on these assessments to ensure that enrollment goals are met efficiently.
In 2025, the emphasis on improving recruitment tactics is crucial, especially as innovative methods are needed to boost participation and inclusivity in research studies. For example, culturally aware outreach has demonstrated a notable increase in recruitment from varied populations, tackling conventional obstacles that have historically restricted access to research studies. As Max Baumann, Head of Execution, noted, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
Furthermore, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a prominent location for medical studies in Latin America, backed by Colombia's Minister of Health. This initiative emphasizes the positive effect of medical studies on local economies, including job creation and healthcare enhancement. Specific metrics from this collaboration indicate a significant reduction in recruitment time and improved retention rates, showcasing the effectiveness of these strategies.
Additionally, Patiro's transparent recruitment plan, where AstraZeneca only pays for eligible patients, exemplifies a contemporary strategy that aligns with the goal of optimizing recruitment processes. By applying these best practices, research teams can enhance their recruitment outcomes and aid in achieving more successful trial results.
Post-Market Clinical Follow-Up: Ensuring Continued Compliance After Approval
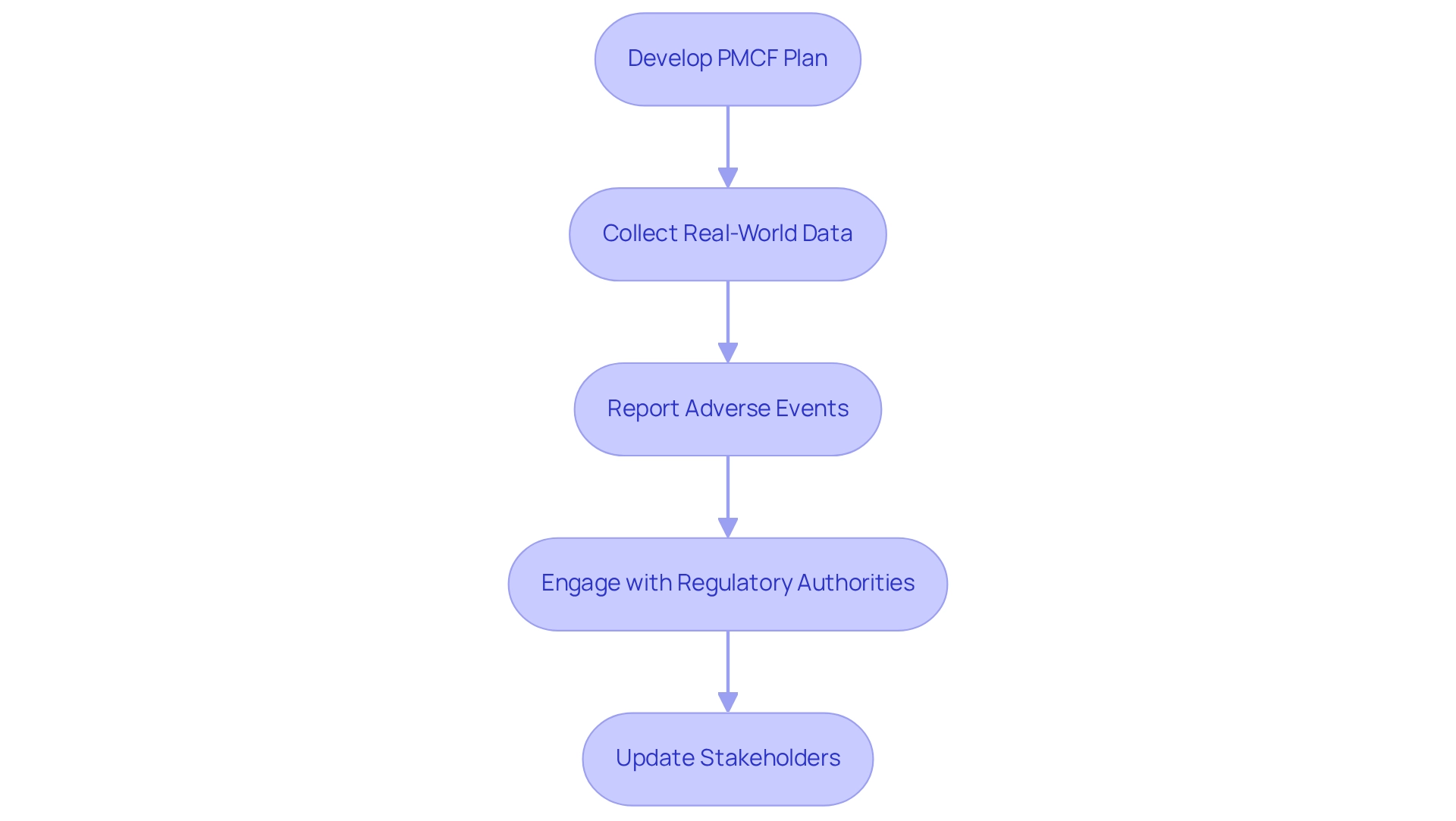
Post-market medical follow-up (PMCF) is an essential component of the regulatory framework governing medical devices, ensuring ongoing compliance and safety after market approval. To effectively implement PMCF, manufacturers should consider the following steps:
- Develop a PMCF Plan: Craft a comprehensive PMCF plan that outlines the methodologies for gathering and analyzing ongoing information. This plan must be dynamic, evolving as new information becomes available, to ensure it remains relevant and effective. Recognizing that the PMS plan is a living document, it should be updated as new information arises.
- Collect Real-World Data: Leverage real-world evidence (RWE) to evaluate the long-term safety and effectiveness of the device across varied patient demographics. For instance, Quinten Medical's retrospective PMCF studies illustrate how RWE can produce robust medical data that meets regulatory expectations, particularly under the Medical Device Regulation (MDR).
- Report Adverse Events: Implement a rigorous system for the timely reporting and management of adverse events or safety issues that may arise post-approval. This proactive approach not only enhances patient safety but also aligns with the requirements set forth by regulatory bodies.
- Engage with Regulatory Authorities: Foster continuous communication with COFEPRIS and other relevant regulatory agencies regarding PMCF outcomes and any necessary corrective actions. This engagement is crucial for maintaining compliance and ensuring that any emerging safety concerns are promptly addressed.
- Update Stakeholders: Regularly communicate with all stakeholders, including healthcare providers and patients, about the device's performance and any pertinent safety information. Keeping stakeholders informed is vital for building trust and ensuring that users are aware of the latest developments regarding the device.
The significance of PMCF in medical device regulation cannot be overstated. It is an ongoing process that not only refreshes the medical assessment but also plays a vital role in post-market monitoring. According to the European Parliament and Council, "PMCF shall be understood to be a continuous process that updates the clinical evaluation referred to in Article 61 and Part A of this Annex and shall be addressed in the manufacturer’s post-market surveillance plan."
Additionally, recent statistics indicate that post-market surveillance (PMS) is mandatory for devices intended for implantation in the human body for over one year, underscoring the necessity of a well-structured PMCF plan. By adhering to these best practices, manufacturers can ensure compliance for clinical trials under COFEPRIS and contribute to the overall safety and efficacy of medical devices in the market. At bioaccess®, we leverage our 20+ years of expertise in managing PMCF studies, Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pivotal Studies to navigate these complexities, ensuring that our clients meet regulatory expectations while maintaining the highest standards of patient safety.
Furthermore, bioaccess® is committed to data protection and has established grievance procedures to address client concerns, reinforcing our dedication to compliance and trust.
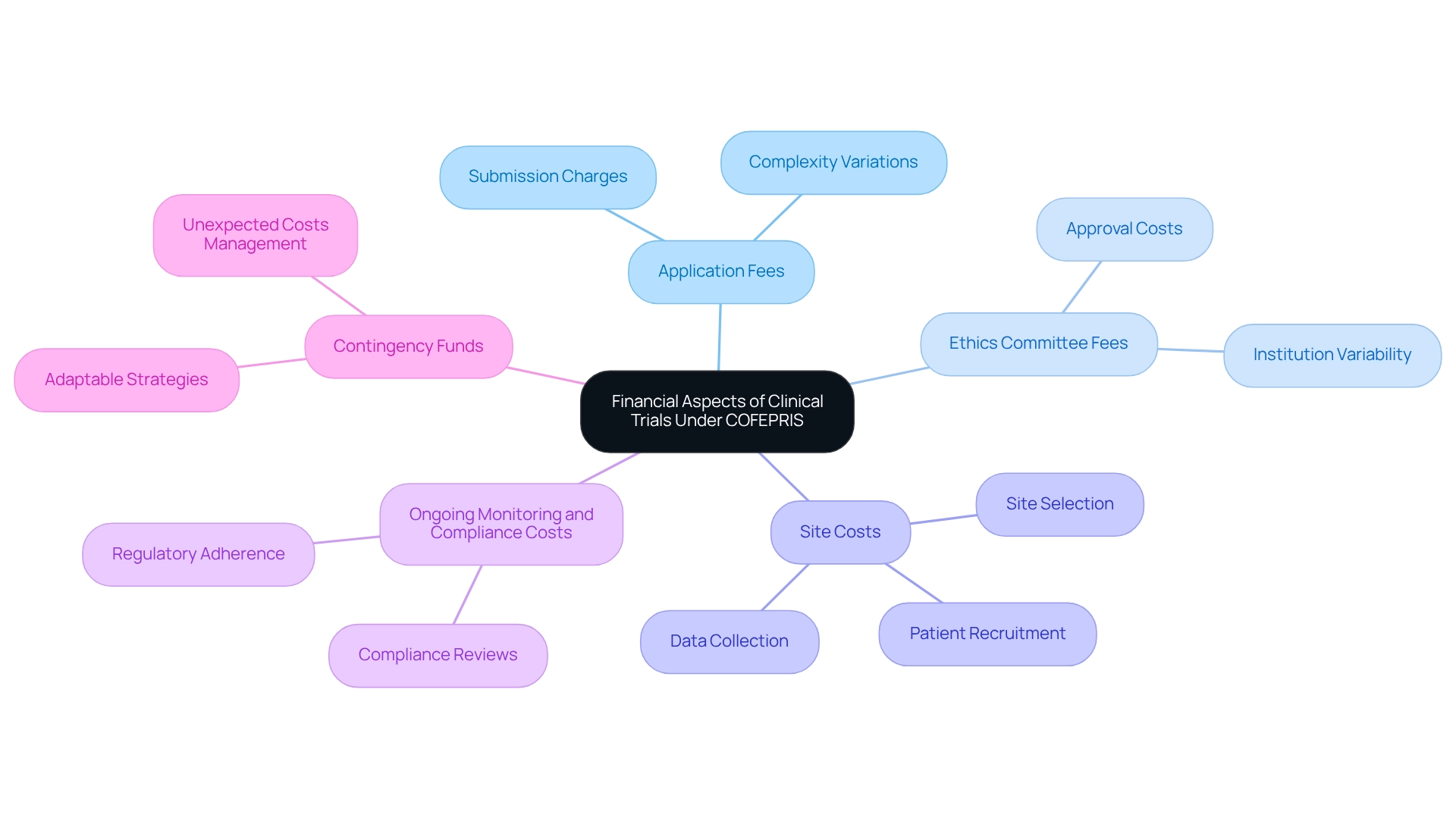
Understanding the Financial Aspects: Fees and Costs of Clinical Trials Under COFEPRIS
Carrying out medical studies in Mexico involves various fees and expenses that organizations must consider, especially when leveraging the extensive management services for medical studies provided by bioaccess®.
Application Fees: COFEPRIS imposes charges for the submission of clinical research applications, which can vary depending on the complexity of the study. With bioaccess®'s expertise, backed by over 20 years of experience in Medtech, organizations can navigate these complexities more effectively.
Ethics Committee Fees: It is essential to budget for fees associated with obtaining approval from ethics committees, which may also vary by institution. Bioaccess® ensures that all ethical considerations are met, streamlining this process with a flexible and customized approach.
Site Costs: Organizations should consider costs related to site selection, including site management, patient recruitment, and data collection. Bioaccess® specializes in site selection and management, optimizing these costs through their extensive network and experience.
Additionally, it is crucial to allocate funds for ongoing monitoring and compliance for clinical trials under COFEPRIS to ensure adherence to regulatory requirements. Bioaccess® provides robust compliance reviews and project management to ensure compliance for clinical trials under COFEPRIS and to mitigate these costs.
Contingency Funds: Allocate contingency funds to manage any unexpected costs that may arise during the process. With bioaccess®'s adaptable strategy, organizations can more effectively foresee and handle these situations. By collaborating with bioaccess®, organizations can benefit from expedited medical device research services in Latin America, ensuring a successful study process while positively influencing local economies through job creation and enhancements in healthcare.
Leveraging Foreign Clinical Data: Opportunities for Streamlined Compliance
Utilizing international research information can greatly simplify compliance for clinical trials under COFEPRIS in Mexico. It is essential to consider the following:
- Data Acceptance: The regulatory authority typically accepts clinical information from international studies conducted in reference nations, significantly accelerating the approval process. This acceptance is crucial as it creates a more efficient pathway to market, particularly for innovative medical technologies.
- Documentation Requirements: It is imperative that all foreign information is meticulously documented and adheres to COFEPRIS standards for quality and reliability. This includes thorough reports outlining the methodology, results, and pertinent statistical analyses, ensuring compliance for clinical trials under COFEPRIS so that the information can withstand regulatory scrutiny. Notably, changes to expiry dates necessitate a stability or aging study report, which is essential for compliance under COFEPRIS.
- Comparative Assessment: A comprehensive comparative assessment is essential to illustrate the significance of international information to the Mexican population and healthcare context. This involves highlighting demographic similarities and potential differences that could impact the efficacy and safety of the medical device or treatment being studied. For instance, a child is defined as under 12 years of age, and adolescents are those between 12 and 18 years of age according to Children, which is crucial for understanding the target demographics in clinical studies.
- Engage Local Experts: Collaborating with local specialists, such as those from bioaccess®, is essential for interpreting foreign information in a manner that aligns with Mexican regulatory expectations. Their insights can help bridge any gaps in understanding and ensure that the data is presented in a context that resonates with the reviewers. With over 20 years of experience in Medtech, bioaccess® specializes in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, providing the expertise needed for successful trial navigation.
- Regulatory Communication: Maintaining open lines of communication with the health authority is essential for ensuring compliance for clinical trials under COFEPRIS. This proactive approach enables clarification of any inquiries concerning the use of foreign information in your application, promoting a smoother review process. Recent developments show promising trends in the acceptance rates of foreign research findings by the regulatory agency, particularly as the organization continues to adapt to global standards. The E22 Guideline, approved by the ICH Assembly in June 2023, highlights the significance of incorporating patient preference information into product development, which corresponds with COFEPRIS's evolving framework for information acceptance.
A case study on pharmacovigilance and safety reporting protocols demonstrates the necessity of following established guidelines to ensure participant safety and effective monitoring of adverse reactions during research studies. This underscores the importance of not only utilizing foreign data but also ensuring that all safety protocols are strictly adhered to in order to maintain compliance and safeguard participants. As Flávia C. Firmino, Sr Director at Pfizer, observes, comprehending regulatory requirements is vital for successful studies in the region.
Special Considerations for Software and Digital Health Applications in Clinical Trials
As digital health technologies increasingly integrate into clinical trials, several critical considerations must be addressed to ensure adherence to regulatory guidelines:
- Regulatory Classification: It is essential to ascertain whether the software qualifies as a medical device under COFEPRIS regulations. This classification significantly influences the approval process, determining the necessary documentation and regulatory pathway. Understanding the regulatory landscape is crucial for successful compliance, particularly in the context of Latin America’s evolving Medtech environment.
- Information Privacy and Security: Adhering to protection regulations is crucial, particularly regarding the handling of sensitive patient details. Statistics show that privacy compliance problems are common in clinical studies, with numerous organizations encountering difficulties in protecting participant information. At bioaccess®, we implement strong privacy measures to comply with legal requirements and safeguard participant information, ensuring client trust and security throughout the study process. Furthermore, our complaint procedures are intended to tackle any issues related to information protection, reinforcing our dedication to transparency and adherence.
- User Experience: The design of software applications should prioritize user experience, ensuring they are intuitive and accessible for participants. For example, the case study of Hotel Castle Salam illustrates how a user-friendly interface can improve participant engagement and information accuracy, ultimately aiding in the trial's success. Smooth incorporation of digital health technologies into medical workflows reduces disruption and improves information collection efficiency, enabling healthcare staff to concentrate on patient care.
- Integration with Medical Workflows: It is essential to assess how the software will integrate into current medical workflows. The incorporation of digital health technologies should be designed to enhance current practices, ensuring that healthcare personnel can efficiently gather and manage data without compromising patient care.
- Continuous Monitoring: Establishing systems for ongoing monitoring of software performance and user feedback is vital. This proactive strategy allows prompt recognition and resolution of problems, ensuring that the software stays efficient and adheres to standards throughout the study.
By tackling these factors, research organizations can maneuver through the intricacies of COFEPRIS regulations while ensuring compliance for clinical trials under COFEPRIS and leveraging the advantages of digital health technologies in their studies. Additionally, as emphasized by Ebix's acknowledgment in Fortune's list of 100 Fastest-Growing Companies, adherence and innovation are crucial for success in the changing environment of medical research. At bioaccess®, we are committed to facilitating medical device clinical trials in Latin America, ensuring comprehensive support from feasibility studies to project management and reporting.
Conclusion
Navigating the regulatory landscape of clinical trials in Mexico, particularly under the auspices of COFEPRIS, is a multifaceted endeavor that demands meticulous planning and execution. This article has examined the essential steps for obtaining clinical trial approval, highlighting the necessity of a structured approach that encompasses:
- Preparing a comprehensive clinical trial application
- Securing ethics committee approval
- Submitting to COFEPRIS with all requisite documentation
Moreover, the significance of ethical considerations and ongoing compliance cannot be overstated. From informed consent to continuous monitoring, these elements are crucial for ensuring participant safety and upholding the integrity of the research process. Additionally, the strategic selection of clinical sites and effective recruitment strategies are pivotal to the success of trials, as evidenced by recent trends that prioritize community engagement and technological integration.
As the demand for innovative medical solutions continues to escalate, understanding the financial implications and leveraging foreign clinical data present further opportunities for streamlined compliance. The commitment to post-market clinical follow-up underscores the necessity of ongoing vigilance in the regulatory process, ensuring that medical devices remain safe and effective long after their initial approval.
In conclusion, the evolving landscape of clinical research in Mexico, shaped by COFEPRIS regulations, necessitates a comprehensive and strategic approach from researchers and sponsors alike. By adhering to established guidelines and embracing ethical practices, the pathway to advancing medical technology and enhancing patient outcomes becomes clearer and more attainable.
Frequently Asked Questions
What is the role of the Federal Commission for Protection against Sanitary Risks (COFEPRIS) in Mexico?
COFEPRIS serves as Mexico's central regulatory authority overseeing research involving human participants, ensuring the security and effectiveness of medical products, and advancing medical research.
Why is understanding COFEPRIS regulations important for researchers and sponsors?
Understanding COFEPRIS regulations is vital for compliance in clinical trials, which includes the approval procedure, oversight of ongoing studies, and adherence to national and international standards.
What recent updates have been made to COFEPRIS's regulatory guidelines?
Recent updates emphasize stringent ethical oversight, especially in studies involving vulnerable populations, ensuring that risks are justified by potential benefits and that strict supervision is in place.
What documentation retention requirements do sponsors have under COFEPRIS?
Sponsors must retain essential documentation for at least five years following product registration or two years after the last marketing approval or formal discontinuation of clinical development.
What steps are involved in securing research study approval from COFEPRIS?
The steps include preparing the Clinical Trial Application (CTA), obtaining approval from an Ethics Committee, filing the CTA with COFEPRIS, and awaiting review.
How long does COFEPRIS typically take to review applications?
COFEPRIS typically examines applications within a maximum of 30 calendar days.
What is the significance of the Research Protocol Authorization Process?
This process requires applicants to secure authorization from COFEPRIS and favorable decisions from both the Research Ethics Committee and the health institution's Research Committee, which can extend approval timelines.
How does bioaccess® assist in the research management process?
bioaccess® provides comprehensive research management services, including feasibility studies, compliance reviews, project management, and ensuring adherence to regulatory standards.
What recent enhancements have been made to the medical research application process?
Enhancements have been made to harmonize technical requirements and adopt ICH guidelines, aimed at improving the efficiency of regulatory processes.
What ethical considerations are highlighted in the context of medical studies?
Ethical considerations focus on participant safety, particularly in emergency situations where investigational drugs may be used without prior consent, provided that ethics committees are informed afterward.
Why are clinical studies important for medical device development?
Clinical studies confirm the safety and effectiveness of medical devices, provide critical information for regulatory submissions, and can expedite approval processes, enhancing patient outcomes.
What is the impact of research studies on local economies?
Research studies contribute to job creation, economic development, and the enhancement of healthcare, reflecting their importance beyond regulatory compliance.




