Overview
The article centers on leveraging local expertise for successful clinical trials in Colombia, underscoring the significance of comprehending the local healthcare landscape, cultural factors, and strategic partnerships. It articulates the advantages of collaborating with regional organizations and utilizing local knowledge to navigate regulatory requirements, enhance patient recruitment, and improve overall study outcomes. This approach positions Colombia as a favorable location for clinical research, highlighting the critical role of collaboration in achieving success in the field.
Introduction
In the rapidly evolving world of clinical research, Colombia is emerging as a pivotal player, boasting a robust landscape for conducting clinical trials. With over 2,000 studies recorded by early 2025, the country showcases its commitment to advancing medical technology and research capabilities, particularly in Phase 3 trials that validate the efficacy and safety of medical devices.
As U.S. companies look to expand their reach in Latin America, understanding the nuances of Colombia's healthcare system, regulatory environment, and socio-economic factors becomes essential. This article delves into the intricacies of conducting clinical trials in Colombia, exploring strategies for:
- Building local partnerships
- Navigating regulatory requirements
- Leveraging technology to enhance trial efficiency and patient engagement
By embracing these elements, stakeholders can unlock the full potential of Colombia's clinical trial landscape, ultimately contributing to improved healthcare outcomes and innovation in the region.
Understanding the Colombian Clinical Trials Landscape
To effectively carry out medical studies in the country, comprehending the existing environment is essential. As of early 2025, Colombia has recorded a total of 2,018 clinical studies, with a notable number reaching completion. The distribution of these trials reveals a strong emphasis on Phase 3 studies, which are crucial for validating the efficacy and safety of medical devices before they reach the market.
A thorough understanding of the local healthcare system is imperative, particularly regarding the roles played by hospitals and clinics, as these institutions are pivotal in forming partnerships and executing recruitment strategies. Additionally, awareness of socio-economic factors that influence patient participation is vital. These factors can significantly impact recruitment and retention rates, ultimately influencing the success of research studies.
Colombia's commitment to improving its medical research capabilities positions it as a key player for U.S. medical device companies seeking to broaden their reach in Latin America. The nation's developing regulatory system, managed by INVIMA, the National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO, along with rising investment in research studies, further emphasizes its potential as a beneficial setting for conducting investigations. For context, Argentina's medical research market is expected to achieve USD 506.1 million by 2030, suggesting considerable growth potential in the area.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked, "I had a positive experience with bioaccess® during its first human study in South America," underscoring the beneficial influence of regional knowledge. Moreover, over the last ten years, annual funding in the research sector in Latin America's Andean Region has surged from $3-4 million to more than $50 million, highlighting the necessity for sponsors to tackle linguistic, cultural, and socio-economic obstacles to enhance patient safety and improve the quality of studies and healthcare in the area.
Cultural differences in patient interactions, such as the tendency to accept physician recommendations without question, complicate the informed consent process in Latin America. By leveraging local expertise for trials in Colombia and understanding the intricacies of the Colombian healthcare environment, sponsors can enhance their research outcomes and address challenges effectively. This is demonstrated by successful partnerships, such as bioaccess™ collaborating with Welwaze Medical Inc. for the Celbrea® medical device launch and GlobalCare Clinical Studies to improve ambulatory services, achieving over 50% reduction in recruitment time and 95% retention rates.
Building Strategic Partnerships with Local Organizations
Leveraging local expertise for trials in Colombia is crucial for the success of clinical studies, necessitating strategic collaborations with regional organizations. Begin by identifying potential collaborators, including local Contract Research Organizations (CROs), hospitals, and academic institutions. Engaging in discussions with these organizations provides valuable insights into their capabilities and how they can align with your project goals.
Establishing transparent communication pathways and clearly outlining roles and responsibilities is essential for fostering successful cooperation.
Colombia presents a particularly advantageous environment for research studies due to its competitive benefits. The nation offers substantial cost reductions—approximately 30% lower than studies conducted in North America or Western Europe—alongside a swift regulatory process, with IRB/EC and MoH (INVIMA) evaluations typically completed within 90-120 days. Furthermore, Colombia's healthcare system is well-respected, ranked #22 by the World Health Organization and recognized for its quality by outlets such as America Economia and International Living.
The inclusion of R&D tax incentives enhances the nation's attractiveness for research studies, allowing small and midsize firms to benefit from a 100% tax deduction, a 25% tax discount, and a 50% tax credit on R&D expenses. This financial support is complemented by a robust patient recruitment landscape, with a population exceeding 50 million and 95% coverage under universal healthcare.
Moreover, forming alliances with patient advocacy groups can significantly enhance recruitment efforts. These groups not only ensure that studies align with patient needs and expectations but also foster community involvement, which is essential for successful study outcomes.
Collaborating with regional CROs, hospitals, and educational institutions in Colombia can yield innovative solutions and improved patient access, ultimately advancing the progress of medical technology in the region.
Recent case studies illustrate this potential, as evidenced by the partnership between bioaccess™ and Caribbean Health Group, which aims to position Barranquilla as a leading site for research studies in Latin America, supported by the nation's Minister of Health. Additionally, the integration of digital technologies within the country's medical research institutions has enhanced communication and collaboration among stakeholders, further emphasizing the importance of regional partnerships in the research process. Notably, the Digital Hospital maintains medical records in a PostgreSQL database for verification and traceability, thereby enhancing the reliability of data management.
Leveraging local expertise for trials in Colombia enables sponsors to navigate the complexities of research studies more efficiently, ensuring timely and favorable outcomes.
Navigating Regulatory Requirements for Clinical Trials
Navigating the regulatory requirements for research studies in Colombia necessitates a thorough understanding of the National Food and Drug Surveillance Institute (INVIMA) and its crucial role in the approval process. Key regulations, particularly Resolution 2378 of 2008, delineate the framework for clinical trial approvals, ensuring compliance with ethical and scientific standards. To commence the process, it is vital to prepare thorough documentation, including the study protocol, informed consent forms, and ethical approvals from regional Institutional Review Boards (IRBs).
Leveraging local expertise for trials in Colombia can significantly streamline this process by engaging local regulatory consultants. Their expertise can provide invaluable guidance on the submission process and help mitigate potential challenges that may arise. Moreover, keeping informed about updates from INVIMA is essential, as regulatory changes can directly affect study timelines and requirements.
The nation's commitment to regulatory excellence, as evidenced by INVIMA's established framework, positions it as an appealing destination for Medtech companies. The regulatory landscape not only ensures patient safety but also aligns with international standards, fostering an environment conducive to innovation and growth. For example, bioaccess, a prominent Contract Research Organization (CRO), provides extensive study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These services are crucial for navigating this environment effectively.
Additionally, Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provides critical insights that enhance the understanding of local regulations.
As pointed out by Moore N., 'the ongoing evolution in monitoring and reporting practices improves research outcomes,' emphasizing the significance of these practices in the realm of regulatory excellence. This evolution is crucial for maintaining high standards in medical research.
In summary, grasping and effectively navigating the regulatory obligations in the country, particularly through the lens of INVIMA's regulations, is essential for the success of research studies. This approach not only enhances compliance but also involves leveraging local expertise for trials in Colombia, which is necessary for advancing medical technologies in a rapidly evolving market. The case study titled 'Conclusion: Colombia's Emergence in the Global Medtech Landscape' further illustrates how the nation's regulatory framework supports Medtech innovation, reinforcing the advantages of conducting research in this region.
Recruiting and Retaining Qualified Research Personnel
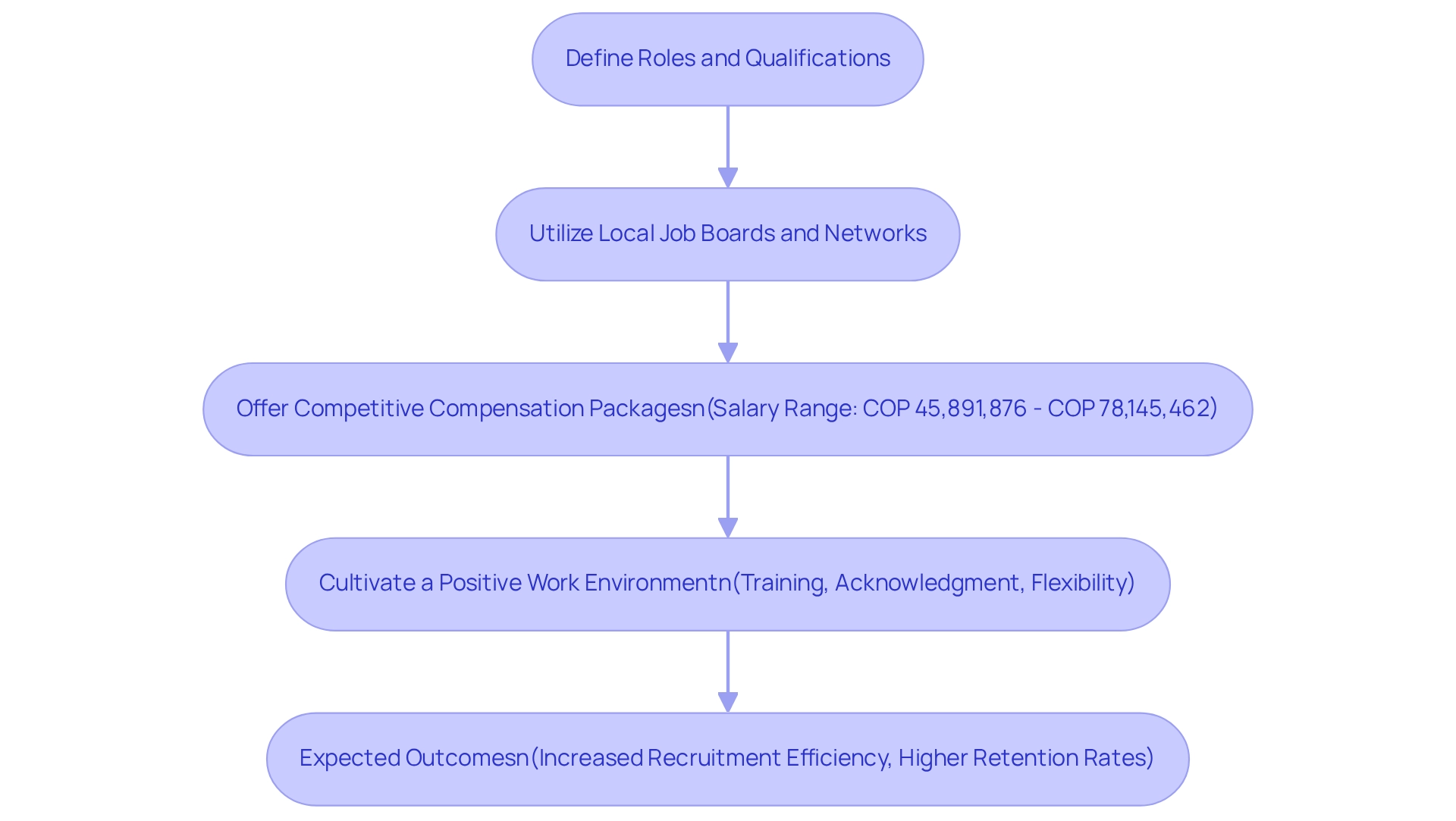
Hiring and retaining skilled research staff is crucial for leveraging local expertise in clinical trials in Colombia. It begins with a clear definition of roles and qualifications necessary for your study, ensuring alignment with the specific demands of the clinical research landscape. To attract a diverse pool of candidates, utilize local job boards, universities, and professional networks.
Offering competitive compensation packages is essential, given that the average salary for researchers in Colombia ranges from COP 45,891,876 to COP 78,145,462. Notably, 25% of Clinical Research Coordinators earn less than COP 41,280,700, while 75% earn above COP 72,240,100, underscoring the importance of competitive salaries in recruitment efforts. Utilizing platforms like ERI SalaryExpert can provide valuable insights into compensation trends, assisting you in assessing and adjusting your salary offerings effectively.
To enhance retention rates, it is vital to cultivate a positive work environment that prioritizes employee well-being and job satisfaction. Opportunities for professional growth, such as frequent training sessions and workshops, can keep your team updated on the latest research practices and regulatory changes. This approach fosters a culture of continuous learning and demonstrates a commitment to staff development, which is essential for retaining skilled personnel. Additionally, implementing best practices for staff retention, such as acknowledging accomplishments and providing flexible work arrangements, can significantly enhance loyalty and reduce turnover within your research teams.
Understanding salary dynamics, as illustrated by the case study comparing public and private sector salaries—where public sector employees earn approximately 11% more than their private sector counterparts—can inform your compensation strategies. By focusing on these approaches and leveraging local expertise for trials in Colombia, including the comprehensive research study management services provided by bioaccess, you can build a strong workforce that drives the success of your research initiatives. The collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies this strategy, achieving over a 50% decrease in recruitment duration and a 95% retention rate, ultimately enhancing ambulatory services for studies in South America.
Furthermore, the economic impact of medical studies on regional economies, including job creation and healthcare improvement, underscores the significance of these initiatives in fostering international cooperation and development.
Cultural Considerations in Patient Recruitment and Engagement
Cultural factors are essential in influencing patient recruitment and engagement approaches for research studies, particularly when leveraging local expertise for trials in Colombia. Understanding regional customs, beliefs, and values is crucial, as these elements significantly impact patient perceptions of healthcare research. Notably, approximately 60% of medical studies conducted in Latin America receive funding from international pharmaceutical firms, which can shape recruitment strategies and participant selection inclusivity.
To effectively engage potential participants, leveraging local expertise for trials in Colombia is vital for developing culturally sensitive recruitment materials that are not only available in regional languages but also resonate with the community's values and traditions. The FDA underscores that inclusion does not automatically equate to having adequate data on patient subgroups for meaningful analysis, emphasizing the necessity of inclusive recruitment practices.
Building trust within the community is paramount. Engaging with community leaders and local groups fosters connections that encourage participation in research studies. The partnership between bioaccess™ and Caribbean Health Group aims to bolster this trust by leveraging local expertise for trials in Colombia, positioning Barranquilla as a key location for research in Latin America, supported by the Minister of Health.
Leveraging local expertise for trials in Colombia enhances credibility and promotes transparent communication about the benefits and processes of medical research. Additionally, participants have expressed a desire to incorporate cultural safety into their professional practices, potentially improving healthcare outcomes. Conducting focus groups can yield valuable insights into the effectiveness of recruitment strategies and materials, ensuring that the approaches employed are relevant and appealing, ultimately leading to enhanced recruitment outcomes.
The case study titled 'Avoiding Confirmation Bias in Research' highlights the importance of maintaining rigorous standards in research practices, aligning with the theme of cultural sensitivity and community involvement. Furthermore, the collaboration with GlobalCare Clinical Studies has demonstrated significant improvements in ambulatory services for research in South America, achieving over a 50% reduction in recruitment time and 95% retention rates. Prioritizing cultural factors and community engagement through local expertise for trials in Colombia can enable research studies in the region to attain higher participation rates and more impactful results. It is also noteworthy that cultural safety training is not yet standard in Colombian medical education, but its inclusion could greatly enhance interactions between health professionals and users of traditional medicine.
Best Practices for Conducting Clinical Trials in Colombia
To achieve successful clinical trials in Colombia, it is essential to implement best practices that enhance both efficiency and data quality. Begin by creating thorough protocols and standard operating procedures (SOPs) tailored to the specific context. These SOPs must reflect the unique cultural and socio-economic landscape, ensuring they resonate with regional healthcare practices and patient expectations.
Leveraging local expertise for trials in Colombia is paramount, as the country boasts over 20 years of experience in training staff in clinical research facilities, significantly enhancing study execution.
Utilizing electronic data capture systems can streamline data collection and management, facilitating real-time access to study data. Regular monitoring of study progress through site visits and audits is critical for ensuring compliance with established protocols and regulatory requirements. This proactive approach not only safeguards compliance but also enhances the overall quality of research outcomes.
Open communication with all stakeholders—including sponsors, investigators, and participants—is vital for swiftly addressing any concerns that may arise during the study. This openness fosters confidence and teamwork, essential elements for effective study execution. Moreover, proactively gathering and integrating patient input can lead to ongoing enhancements in study procedures and participant experiences.
Cultural differences can significantly influence patient interactions with doctors, impacting informed consent procedures; therefore, it is crucial to consider these factors in study design. By tailoring research designs to align with regional healthcare practices, as illustrated in relevant case studies, the significance of findings for diverse populations is improved, ultimately enhancing the quality of medical research results. As the landscape of outsourced research studies evolves, leveraging local expertise for trials in Colombia will be critical, alongside utilizing local knowledge and alliances, such as collaborating with reliable CROs like bioaccess®, to navigate the challenges posed by linguistic and cultural variances, thereby ensuring participant safety and the integrity of the study.
Collaborating with established organizations can expedite the research process, ensuring adherence to protocols and improving overall research effectiveness. Additionally, bioaccess® offers extensive research study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—elements essential for the successful execution of any study. Understanding the regulatory roles of INVIMA, the National Food and Drug Surveillance Institute, is also vital for effectively maneuvering through the research landscape.
Furthermore, the impact of medical studies on local economies, such as job creation and healthcare enhancement, underscores the significance of these assessments in fostering economic development and global cooperation.
Leveraging Technology for Efficient Clinical Trials
In Colombia, leveraging local expertise for trials is essential for conducting effective clinical studies. The implementation of electronic data capture (EDC) systems can significantly streamline data collection processes, minimizing errors and enhancing data integrity. These systems not only facilitate real-time data access but also align with the growing trend towards patient-centric studies, which prioritize the experiences and needs of participants.
With over 20 years of expertise in the Medtech field, bioaccess® is well-positioned to guide organizations in adopting these technologies effectively. The adoption of telemedicine platforms represents another transformative approach, enabling remote patient monitoring and consultations. This technology improves patient engagement and enhances retention rates, as participants can easily access care without the need for frequent in-person visits. Additionally, mobile applications can be employed for patient recruitment and continuous communication, offering real-time updates and feedback that foster a more engaging research environment.
Dr. Lisa A. Marsch, a lead researcher associated with the mobile intervention platform utilized in this study, highlights the significance of such technologies in improving study results. Educating your research team on these technologies is crucial to ensure their efficient application during the research process. By equipping staff with the necessary skills, you can maximize the benefits of these innovations, ultimately leading to improved outcomes and heightened participant satisfaction. Recent research using electronic health records (EHR) from 49 healthcare institutions throughout the country has provided valuable insights into healthcare practices and patient outcomes, illustrating that the incorporation of technology in medical studies is not only advantageous but increasingly essential to keep pace with changing industry standards and regulatory reforms.
Moreover, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). The organization is actively partnering with Caribbean Health Group to establish Barranquilla as a premier location for medical studies in Latin America, leveraging local expertise for trials in Colombia with the support of Colombia's Minister of Health. This collaboration seeks to enhance the research landscape, contributing to job creation, economic development, and improved healthcare outcomes in the area.
The anticipated results of this partnership encompass expanded research opportunities, improved local knowledge, and a reinforced healthcare system, ultimately benefiting both the local economy and patient access to cutting-edge medical technologies by leveraging local expertise for trials in Colombia.
Future Trends in Clinical Trials in Colombia
Looking ahead, leveraging local expertise for trials in Colombia stands out as a crucial trend poised to significantly influence the future of medical studies. The growing acceptance of decentralized research studies is expected to markedly enhance patient access and involvement, particularly in rural regions where traditional study sites may be limited. This transition not only broadens participation but also effectively addresses the logistical challenges frequently encountered in these areas.
However, the research landscape in Latin America is not without its challenges. Regulatory complexities, recruitment obstacles, logistical hurdles, and cultural considerations can hinder progress. As Roshan Deshmukh astutely observed, "The future of medical exploration in Latin America is bright, with unique opportunities for innovation that can address both local and global health needs."
This perspective underscores the potential to navigate these challenges through innovative approaches, such as the comprehensive research management services provided by bioaccess™, which encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Moreover, the integration of artificial intelligence and machine learning into study design and data analysis is set to revolutionize the clinical research environment. These technologies will streamline processes, enhance data accuracy, and improve decision-making capabilities, ultimately leading to more efficient evaluations. For instance, leveraging extensive operational experience in decentralized studies, the Patient Engagement Suite Technology has proven its effectiveness in enhancing patient recruitment and monitoring, thus improving enrollment accuracy and increasing patient participation.
As regulatory frameworks continue to evolve, it is imperative for organizations to stay vigilant and informed about these changes to ensure compliance. This adaptability will be vital in managing the complexities of conducting medical research in a dynamic environment, particularly under the oversight of INVIMA, the National Food and Drug Surveillance Institute, which plays a critical role in regulatory functions and medical device oversight.
Additionally, prioritizing the development of diverse patient populations is essential. With 30% of the population in Latin America reportedly under 14 years old, ensuring that study participants reflect a broad range of demographics will enhance the generalizability of results, making them more applicable to the wider population. By focusing on these trends and leveraging local expertise for trials in Colombia, including the collaboration between bioaccess™ and the Caribbean Health Group, organizations can strategically position themselves for success in the rapidly evolving landscape of clinical trials.
Conclusion
Understanding the complexities of conducting clinical trials in Colombia is essential for stakeholders eager to leverage the country’s robust healthcare system and regulatory environment. With over 2,000 clinical studies recorded as of early 2025, Colombia has established itself as a key player in the global Medtech landscape, particularly in Phase 3 trials that validate medical devices. The insights gained from building local partnerships, navigating regulatory requirements, and leveraging technology not only contribute to the success of clinical trials but also foster innovation and improved healthcare outcomes in the region.
The importance of strategic collaborations cannot be overstated. Engaging with local organizations, including Contract Research Organizations and patient advocacy groups, enhances recruitment efforts and fosters a community-oriented approach to clinical research. Meanwhile, understanding the regulatory landscape governed by INVIMA ensures compliance and facilitates smoother trial processes, thereby enhancing overall efficiency. As technology continues to reshape the clinical trial landscape, the adoption of electronic data capture systems and telemedicine can significantly improve patient engagement and data integrity.
As the future unfolds, Colombia's clinical trial environment is poised for further growth, driven by trends such as decentralized trials and the integration of artificial intelligence. By prioritizing cultural considerations and embracing innovative practices, stakeholders can navigate challenges effectively and unlock the full potential of clinical trials in Colombia. This commitment to collaboration, compliance, and technological advancement will ultimately lead to better healthcare solutions and a thriving clinical research ecosystem in Latin America.
Frequently Asked Questions
How many clinical studies have been recorded in Colombia as of early 2025?
As of early 2025, Colombia has recorded a total of 2,018 clinical studies.
What type of clinical studies are most emphasized in Colombia?
There is a strong emphasis on Phase 3 studies, which are crucial for validating the efficacy and safety of medical devices before they reach the market.
Why is understanding the local healthcare system important for conducting medical studies in Colombia?
A thorough understanding of the local healthcare system is imperative because hospitals and clinics play pivotal roles in forming partnerships and executing recruitment strategies. Additionally, awareness of socio-economic factors influencing patient participation is vital for recruitment and retention rates.
What role does INVIMA play in Colombia's medical research?
INVIMA, the National Food and Drug Surveillance Institute, manages Colombia's developing regulatory system and is recognized as a Level 4 health authority by PAHO/WHO.
What financial incentives are available for research and development in Colombia?
Colombia offers R&D tax incentives, including a 100% tax deduction, a 25% tax discount, and a 50% tax credit on R&D expenses, which enhance the nation's attractiveness for research studies.
How does Colombia's healthcare system rank globally?
Colombia's healthcare system is well-respected and ranked #22 by the World Health Organization.
What are the advantages of conducting clinical studies in Colombia compared to North America or Western Europe?
Colombia offers substantial cost reductions—approximately 30% lower than studies conducted in North America or Western Europe—and a swift regulatory process, with IRB/EC and MoH (INVIMA) evaluations typically completed within 90-120 days.
How can collaboration with local organizations improve clinical study outcomes in Colombia?
Collaborating with local Contract Research Organizations (CROs), hospitals, and academic institutions can provide valuable insights, enhance recruitment efforts, and yield innovative solutions, ultimately advancing medical technology in the region.
What impact do cultural differences have on the informed consent process in Latin America?
Cultural differences, such as the tendency to accept physician recommendations without question, complicate the informed consent process in Latin America.
What recent partnerships illustrate the potential for research studies in Colombia?
Successful partnerships, such as bioaccess™ collaborating with Welwaze Medical Inc. and Caribbean Health Group, demonstrate the potential for research studies in Colombia, highlighting the importance of regional expertise and collaboration.




