Overview
Navigating regulatory challenges in Latin America for First-in-Human (FIH) and Early-Phase Studies (EFS) requires a thorough understanding of local regulations, collaboration with regional experts, and strategic planning to optimize trial execution. The article emphasizes that leveraging partnerships with Contract Research Organizations (CROs) and conducting detailed feasibility studies can significantly enhance compliance and streamline the approval process, ultimately improving the quality and efficiency of clinical research in the region.
Introduction
The regulatory framework governing clinical trials in Latin America presents a multifaceted challenge, shaped by the distinct rules and guidelines of each country. As the region gains prominence in global clinical research, understanding the specific regulations set forth by local health authorities—such as ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia—becomes increasingly critical.
Colombia, in particular, has distinguished itself as a competitive hub for clinical trials, offering significant cost advantages, expedited approval processes, and a robust healthcare system.
This article delves into the intricacies of the regulatory landscape, common challenges faced in First-In-Human (FIH) and Early Phase Studies (EFS), and best practices for navigating the complexities of clinical research in this dynamic region.
By exploring the strategic partnerships and emerging trends that are shaping the future of clinical trials in Latin America, this discussion aims to provide valuable insights for researchers and stakeholders looking to optimize their operations and enhance compliance within this evolving environment.
Understanding the Regulatory Landscape for Clinical Trials in Latin America
The oversight environment for medical studies in Latin America is a intricate structure shaped by the distinct regulations and protocols of each nation. Understanding the regulations set forth by local health authorities—such as ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia—is essential for the successful execution of trials. Colombia, in particular, has emerged as a powerhouse in medical research within Latin America, thanks to its competitive advantages:
- Significant cost savings of over 30% compared to North America or Western Europe.
- Expedited regulatory processes with IRB/EC and MoH (INVIMA) approvals taking only 90-120 days.
- A robust healthcare system ranked among the best globally.
Hospitals in Colombia are required to undergo a rigorous ICH/GCP certification process to conduct research, ensuring high-quality standards. The collaboration between bioaccess™ and the Caribbean Health Group aims to position Barranquilla as the premier destination for clinical trials, further supported by Colombia's Minister of Health. Effective patient recruitment is bolstered by a population of over 50 million, with 95% covered by universal healthcare.
Additionally, R&D tax incentives, including:
- A 100% tax deduction
- A 25% tax discount
- A 50% future tax credit
- Approximately $10 million in government grants for innovation projects
enhance the investment environment. Understanding local regulations is crucial when navigating regulatory challenges in Latin America for FIH and EFS studies, as it not only streamlines applications but also mitigates potential delays. Moreover, as Latin America is expected to have 90% of its population residing in urban regions by 2050, the chances for strong subject recruitment and retention will keep increasing, shaped by the changing oversight environment.

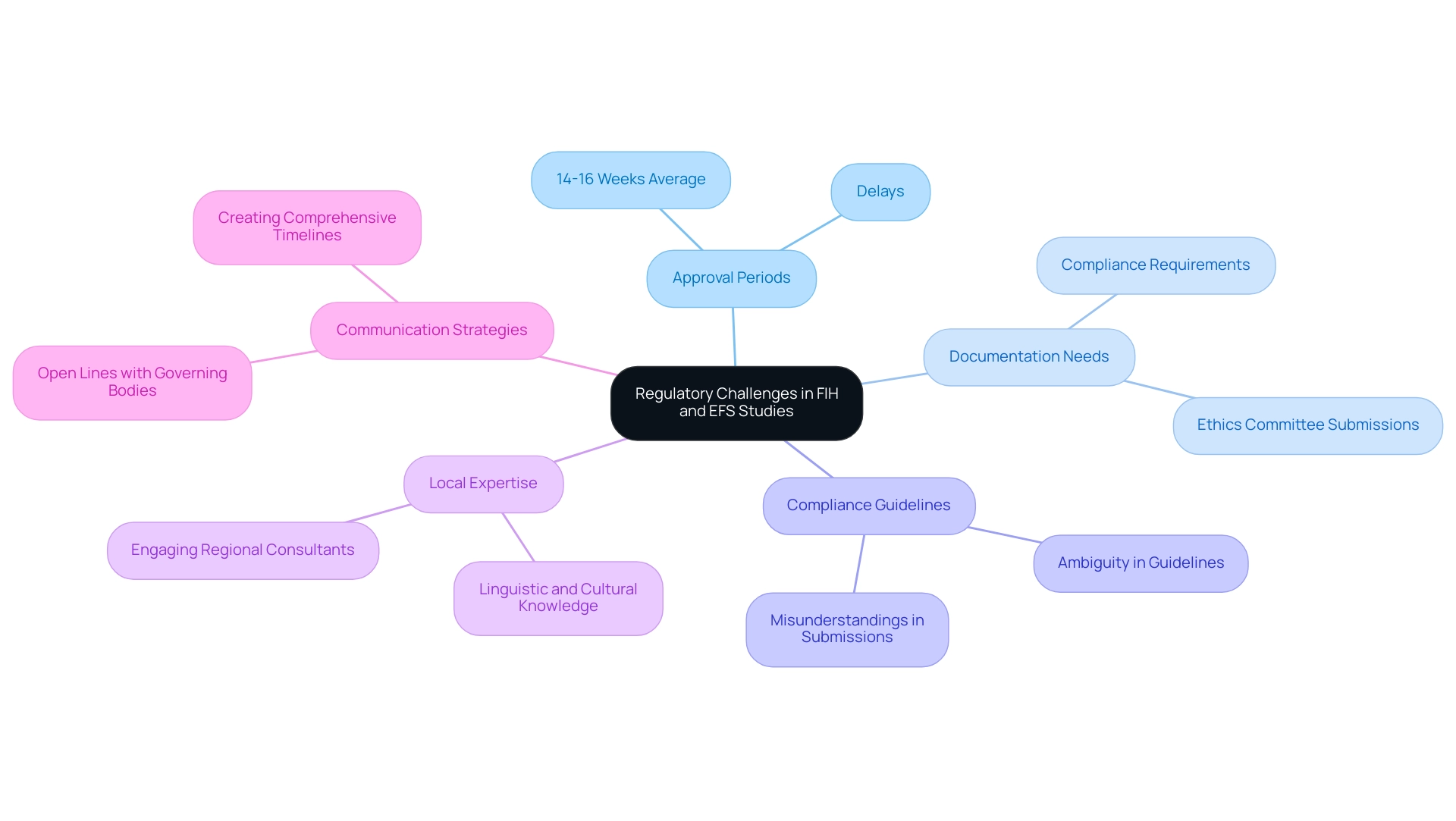
Navigating Common Regulatory Challenges in FIH and EFS Studies
In the domain of medical studies in Latin America, researchers are often challenged by navigating regulatory challenges in Latin America for FIH and EFS studies, including lengthy approval periods and various documentation needs. As Kendle, a CRO specializing in clinical trial management, observes, the average approval times extend from 14 to 16 weeks, highlighting the need for a strategic approach to navigation. One prevalent issue is the ambiguity surrounding compliance guidelines, which can result in misunderstandings and incomplete submissions.
To effectively overcome these challenges, engaging regional regulatory consultants who possess expertise in navigating regulatory challenges in Latin America for FIH and EFS studies is highly advisable. This local knowledge can facilitate more precise submissions and enhance compliance with regional regulations. Furthermore, addressing linguistic and cultural differences is crucial; as highlighted in the case study on language barriers and translation in clinical research, proper translation is essential for informed consent and ethical treatment of participants.
This is where Language Connections comes into play, offering a network of linguists with expertise in various industries to ensure that documentation meets industry standards and compliance requirements. Additionally, our service capabilities include:
- Feasibility and selection of research sites and principal investigators
- Comprehensive review and feedback on study documents to comply with country requirements
- Trial setup and approval processes (including ethics committee and health ministry)
- Import permits for investigational devices
- Thorough study project management and monitoring, including detailed reporting on study status, inventory, and adverse events
Fostering open lines of communication with governing bodies is vital in streamlining interactions and expediting the approval process.
Creating a comprehensive timeline that anticipates possible delays is equally essential for managing expectations and ensuring that studies progress as planned. By addressing these strategies, researchers can focus on navigating regulatory challenges in Latin America for FIH and EFS studies, optimize their operational efficiency, and leverage the expertise of leaders like Ana Criado, Director of Affairs, and Katherine Ruiz, a specialist in compliance for medical devices.
Leveraging Partnerships to Overcome Regulatory Hurdles
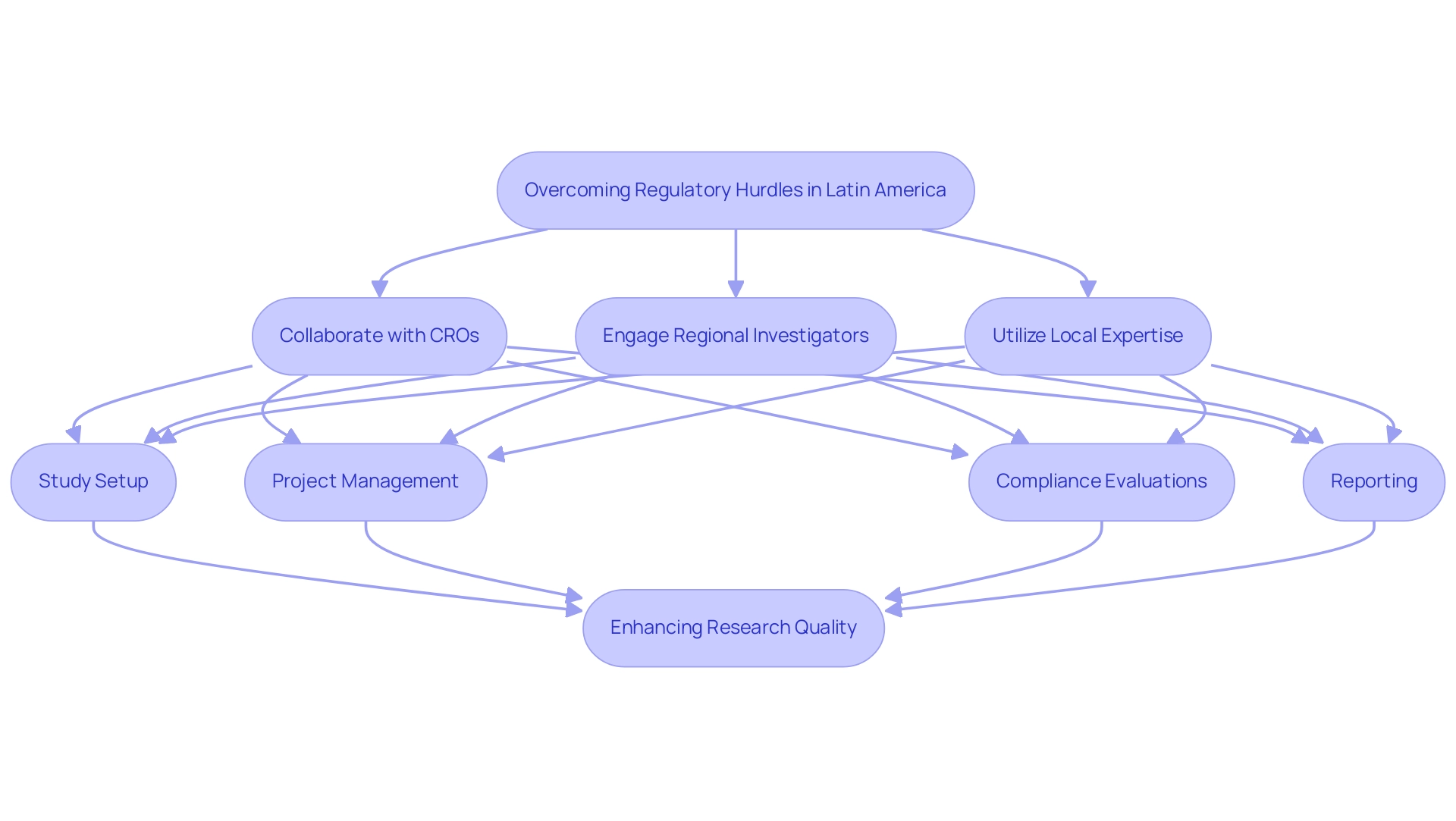
Collaborating with Contract Research Organizations (CROs) that have a strong presence in Latin America is crucial for navigating regulatory challenges in Latin America for FIH and EFS studies. These organizations typically boast well-established connections with nearby governing bodies, facilitating the preparation of submissions that adhere to all necessary guidelines. Moreover, involving regional investigators is crucial, as their comprehension of cultural and compliance nuances can greatly enhance the credibility of clinical studies.
This is clear from the experiences shared by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during the first human test of bioaccess® in Colombia, emphasizing the significance of collaboration with regional expertise. Such regional understanding not only improves interactions with oversight organizations, including INVIMA, the Colombia National Food and Drug Surveillance Institute, but also cultivates trust within the community—essential for patient recruitment and retention. This is especially significant considering the 'recruitment crisis' highlighted by Julio G. Martinez-Clark, CEO of bioaccess, emphasizing the necessity for local expertise in addressing obstacles encountered in research.
By utilizing these strategic alliances, researchers can skillfully manage regulatory obstacles while navigating regulatory challenges in Latin America for FIH and EFS studies and concurrently enhancing the overall quality and adherence of their research. The extensive service capabilities of CROs, including:
- Study setup
- Project management
- Compliance evaluations
- Reporting
further improve the effectiveness of research studies. Furthermore, the significant $1.2 billion investment in Latin America's MedTech sector indicates an increasing acknowledgment of the advantages that CRO partnerships and local investigator participation can provide, particularly in tackling deficiencies like those observed in Haiti, where there are presently no ongoing cancer research studies.
This collaborative approach is increasingly recognized as a best practice in the region.
Best Practices for Conducting FIH and EFS Studies in Latin America
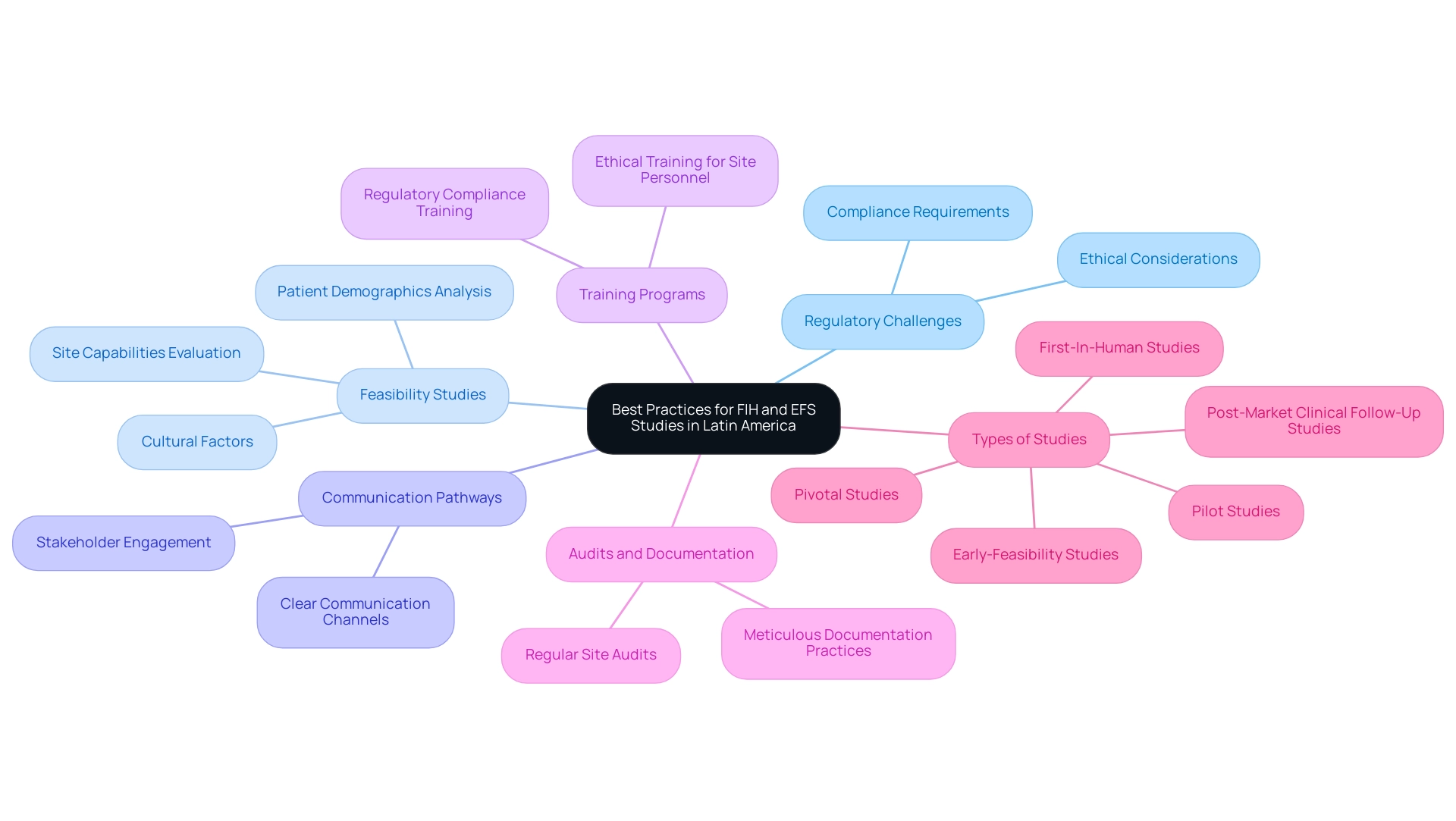
To ensure the successful execution of First-in-Human (FIH) and Early-Phase Studies (EFS) in Latin America, researchers must focus on navigating regulatory challenges in Latin America for FIH and EFS studies through meticulous planning and preparation. Conducting detailed feasibility studies is essential to evaluate site capabilities and patient demographics effectively. These studies not only inform the selection of appropriate sites but also help in understanding cultural and socio-economic factors that can affect patient recruitment and retention.
As noted by Gotuzzo, a leading authority in research, 'the region presents both constraints and opportunities that must be navigated strategically.' At bioaccess®, we utilize over 20 years of expertise in managing clinical research, focusing on:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
Our customized approach ensures that we tailor our services to meet the unique needs of each trial, enhancing efficiency and effectiveness.
Creating clear communication pathways among all stakeholders—including authorities like INVIMA, sponsors, and site personnel—is essential for navigating regulatory challenges in Latin America for FIH and EFS studies and promptly addressing any emerging issues. Implementing comprehensive training programs for site personnel on navigating regulatory challenges in Latin America for FIH and EFS studies, as well as on regulatory compliance and ethical considerations, will significantly enhance the quality of data collected, fostering adherence to established protocols. Moreover, regular audits of study sites and meticulous documentation practices are essential elements that contribute to the overall success of research endeavors.
Significantly, Phase III studies represented a substantial 53.28% revenue share in 2023, highlighting the importance of this segment in the context of research in Latin America. As the CRO market in Mexico and Brazil continues to expand, emphasizing best practices in trial management, the case study on CRO market growth underscores the practical implications of these strategies and strengthens the argument regarding the region's increasing significance in the global trials landscape.
Future Trends in Regulatory Practices for Clinical Research in Latin America
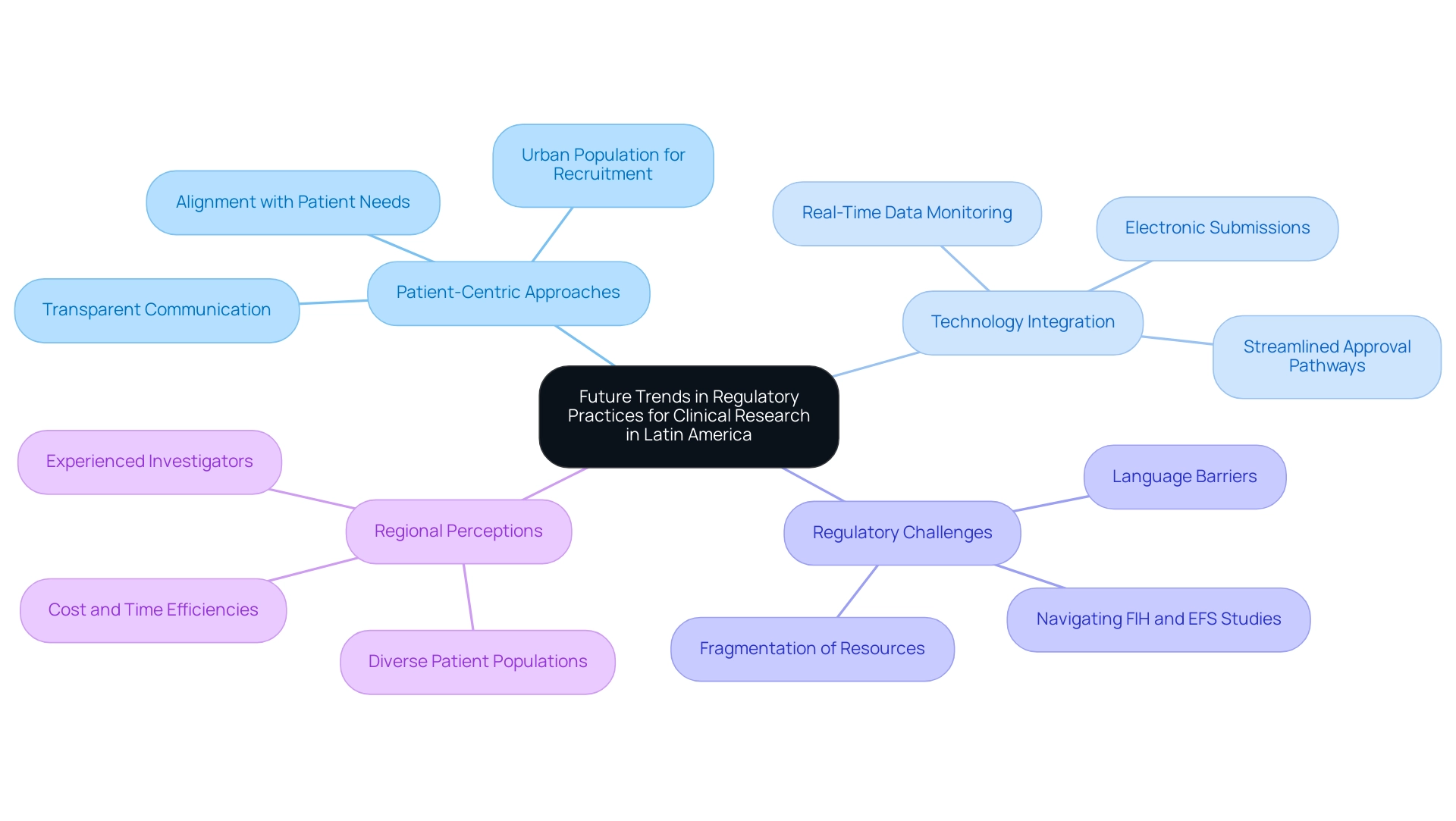
The evolving landscape of medical research in Latin America is marked by several transformative trends that emphasize navigating regulatory challenges in Latin America for FIH and EFS studies. A significant shift towards patient-centric approaches is becoming apparent, with an emphasis on transparent communication with stakeholders influencing how research studies are designed and executed. This trend aligns with the broader global movement towards enhancing the patient experience in clinical trials, with 80% of Mexico's population living in urban areas, facilitating easier recruitment and follow-up.
The integration of advanced technology in oversight processes—such as electronic submissions and real-time data monitoring—promises to streamline approval pathways and bolster compliance. As observed by Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, 'Having a local presence and leadership that is based in the LATAM region strengthens the relationships that Parexel builds with local talent, regulatory authorities based in the area, local associations, and the global network of sites overall.' The case study titled 'Transforming Perceptions of Latin America in Research' emphasizes how the region, previously scrutinized for adhering to U.S. and European standards, is now acknowledged for its cost and time efficiencies, diverse patient populations, and seasoned investigators, making it a favored site for studies.
Furthermore, the increasing emphasis on navigating regulatory challenges in Latin America for FIH and EFS studies could simplify the execution of multinational studies. As Dr. Ana Lucia, an expert in trials, states, 'Adopting patient-centric models not only enhances recruitment but also ensures that trials are more aligned with patient needs.' Furthermore, Medtech companies must navigate challenges such as language barriers and the fragmentation of resources, which can complicate collaboration with regional entities.
Staying abreast of these trends will empower researchers to adapt their strategies proactively, thereby maintaining a competitive edge in the dynamic clinical research landscape while navigating regulatory challenges in Latin America for FIH and EFS studies and addressing the challenges posed by regulatory hurdles and the need for collaboration between Medtech companies and local entities. Services such as feasibility studies, site selection, compliance reviews, and project management play a crucial role in this evolving landscape.
Conclusion
The regulatory landscape for clinical trials in Latin America presents both significant opportunities and challenges for researchers and stakeholders. Colombia stands out as a competitive hub, characterized by its streamlined approval processes, cost advantages, and a robust healthcare system. Understanding the specific regulations set by local health authorities is crucial for navigating the complexities of First-In-Human (FIH) and Early Phase Studies (EFS).
Researchers face common hurdles such as:
- Protracted approval timelines
- Diverse documentation requirements, often exacerbated by regulatory ambiguities
Engaging local regulatory consultants and fostering open communication with authorities can aid in overcoming these challenges, ensuring compliance and enhancing operational efficiency. The importance of local expertise cannot be overstated, as it plays a vital role in building trust within communities and facilitating patient recruitment.
Strategic partnerships with Contract Research Organizations (CROs) can further enhance the ability to navigate regulatory hurdles, leveraging their established relationships and local knowledge. Best practices, including:
- Detailed feasibility studies
- Comprehensive training for site personnel
are essential for successful trial execution. As the region evolves, the integration of patient-centric approaches and advanced technology will continue to reshape regulatory practices, aligning clinical trials with the needs of diverse populations.
In summary, a thorough understanding of the regulatory framework, coupled with effective strategies and partnerships, is key to optimizing clinical research in Latin America. By staying informed of emerging trends and best practices, researchers can enhance their compliance efforts and contribute to the region's growing prominence in the global clinical trials landscape.
Frequently Asked Questions
What is the oversight environment for medical studies in Latin America?
The oversight environment is a complex structure influenced by the unique regulations and protocols of each country, such as ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia.
What advantages does Colombia offer for medical research?
Colombia provides significant cost savings (over 30% compared to North America or Western Europe), expedited regulatory processes (IRB/EC and MoH approvals taking 90-120 days), and a robust healthcare system ranked among the best globally.
What is required for hospitals in Colombia to conduct research?
Hospitals must undergo a rigorous ICH/GCP certification process to ensure high-quality standards in research.
How does patient recruitment in Colombia benefit medical studies?
Colombia has a population of over 50 million, with 95% covered by universal healthcare, enhancing effective patient recruitment.
What R&D tax incentives are available in Colombia?
Incentives include a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants for innovation projects.
Why is understanding local regulations important for conducting studies in Latin America?
It streamlines applications and mitigates potential delays, facilitating smoother navigation of regulatory challenges for First-in-Human (FIH) and Early Phase Studies (EFS).
What challenges do researchers face when navigating regulatory processes in Latin America?
Researchers often encounter lengthy approval periods, various documentation needs, and ambiguity surrounding compliance guidelines.
How can researchers overcome regulatory challenges in Latin America?
Engaging regional regulatory consultants with local expertise can enhance compliance and improve submission accuracy.
Why is addressing linguistic and cultural differences important in clinical research?
Proper translation is essential for informed consent and the ethical treatment of participants, as highlighted by the case studies on language barriers.
What services does Language Connections provide to support clinical trials?
They offer feasibility and selection of research sites, comprehensive review of study documents, trial setup and approval processes, import permits for investigational devices, and thorough project management and monitoring.
What strategies can researchers use to streamline the approval process?
Fostering communication with governing bodies and creating a comprehensive timeline to anticipate possible delays are essential strategies for managing expectations and ensuring studies progress smoothly.

