Overview
Navigating regulatory requirements for medical devices in Latin America involves understanding the specific frameworks of each country, such as ANVISA in Brazil, ANMAT in Argentina, and COFEPRIS in Mexico, which dictate distinct classification, documentation, and approval processes. The article emphasizes the importance of engaging local regulatory experts and maintaining compliance with ongoing surveillance and reporting to ensure successful market entry and patient safety.
Introduction
In the intricate landscape of medical device regulation in Latin America, understanding the specific requirements and processes is paramount for successful market entry. Brazil, with its robust regulatory framework governed by the Agência Nacional de Vigilância Sanitária (ANVISA), presents unique challenges and opportunities for manufacturers.
From classifying devices based on risk to preparing meticulous documentation and ensuring compliance with Good Manufacturing Practices, the journey requires a strategic approach. Moreover, the necessity for ongoing post-market surveillance and engagement with local regulatory experts cannot be overstated.
As the region evolves, staying informed about regulatory updates and leveraging local insights becomes crucial for navigating this complex environment and achieving compliance.
Understanding Brazil's Medical Device Regulatory Framework
Navigating Brazil's medical equipment approval framework necessitates a comprehensive understanding of the Agência Nacional de Vigilância Sanitária (ANVISA), the key authority overseeing the authorization of medical products. The first step is to accurately determine the classification of your equipment, which significantly influences the regulatory pathway. Medical instruments in Brazil are categorized into four classes (I, II, III, and IV) based on their associated risk, with Class I representing the lowest risk and Class IV denoting the highest.
Notably, Class III and IV products require registration with ANVISA, and this authorization is valid for ten years. Each classification entails specific documentation requirements and approval processes. For successful registration, it is essential to prepare detailed technical files, encompassing safety and efficacy data, and submit these to ANVISA for a thorough review.
Furthermore, adherence to Brazilian Good Manufacturing Practices (BGMP) is critical; as noted by expert Margret Seidenfaden, 'If your GMP certificate loses its validity, the device registration will also become invalid!' Additionally, ANVISA's oversight agenda includes ongoing reviews of pharmacovigilance and CBD regulations, highlighting the dynamic nature of the framework.
To illustrate the importance of compliance, consider the comprehensive clinical trial management services provided by bioaccess®, which include:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Their services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all customized to navigate the legal landscape effectively. This essential understanding is crucial for successfully navigating regulatory requirements for medical devices in Latin America, especially when introducing your healthcare product within Brazil’s intricate compliance landscape. Partnering with local specialists, like Katherine Ruiz, who focuses on compliance matters for healthcare products and in vitro diagnostics in Colombia, can further improve your likelihood of adherence and achievement.
Navigating the Regulatory Landscape Across Latin America
Successfully navigating regulatory requirements for medical devices in Latin America, a region with an estimated population of 657 million people as of 2024, necessitates a thorough understanding of the specific requirements in each target country. For example:
- Argentina is overseen by ANMAT.
- Mexico depends on COFEPRIS, each having distinct procedures and documentation requirements.
- In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in supervising health equipment, ensuring adherence to health regulations.
Grasping the extensive method for promoting medical device trials encompasses:
- Site feasibility
- Investigator selection
- The steps for securing clinical trial approval, which involves both IRB/EC endorsement and INVIMA's oversight alongside the MinCIT import permit.
This procedure also encompasses review and feedback on study documents to comply with country requirements, as well as reporting on study status, inventory, and serious and non-serious adverse events. It is essential to devise a clear strategy for compliance submissions, which should include detailed timelines and suitable resource allocation.
Interacting with local compliance consultants can provide essential insights into country-specific nuances, facilitating a more efficient approval process. Additionally, it’s important to be aware of the potential for harmonization among these nations, as certain regions may adopt similar standards. According to the case study titled 'Stay Compliant: Medical Device Industry 2024 Compliance Update,' stakeholders can benefit from understanding the evolving oversight landscape to ensure adherence.
By staying informed about regional trends and policy updates, stakeholders can significantly enhance their capacity for navigating regulatory requirements for medical devices in Latin America, ensuring compliance and expediting market entry. As Guillaume Corpart, CEO and founder of Global Health Intelligence, states, 'This powers MedTech Outlook: Latin America 2024 with fresh, direct data, that is essential to developing growth strategies.' This viewpoint highlights the significance of utilizing precise data in navigating the compliance landscape.
To discuss how we can assist you in this process, BOOK A MEETING today.

Preparing Required Documentation for Regulatory Submission
When preparing the necessary documentation for official submission in Brazil, navigating regulatory requirements for medical devices in Latin America is essential, as it involves compiling a comprehensive technical file that includes critical elements such as:
- Description of the product
- Intended use
- Design specifications
This file must also encompass clinical data that demonstrates the safety and efficacy of the apparatus, alongside detailed risk management reports and appropriate labeling information. Navigating regulatory requirements for medical devices in Latin America necessitates adhering strictly to the guidelines set forth by ANVISA, which includes formatting all documents according to their specific requirements.
Notably, requests for new registration by the supporting institution are prohibited within a period of 12 months after cancellation, which can significantly impact submission timing. Additionally, sponsor-specific essential documents must be retained for at least two years after the last marketing application approval or discontinuation of clinical development, underscoring the importance of documentation retention. A thorough review of the documentation is advisable for navigating regulatory requirements for medical devices in Latin America to identify any potential gaps or inconsistencies prior to submission.
Involving compliance advisors, such as Katherine Ruiz, who specializes in compliance matters for healthcare products and in vitro diagnostics in Colombia, can provide valuable insights and knowledge, ensuring that all documentation meets required standards. As Luca Salvatore pointed out, 'Manufacturers who wish to introduce healthcare products in the EU must adhere to the European Medical Product Regulation MDR,' emphasizing the importance of following comparable compliance standards in Brazil. Furthermore, ethical considerations are paramount; in emergency situations where obtaining signed informed consent is not feasible, the consent of a legal representative or guardian should be sought, as emphasized in the case study titled 'Emergency Situations and Consent.'
By taking these steps and leveraging comprehensive clinical trial management services—including trial set-up, start-up, approval processes, import permits, and project management—sponsors can enhance their chances of a successful submission to authorities.
Understanding Post-Market Surveillance Requirements
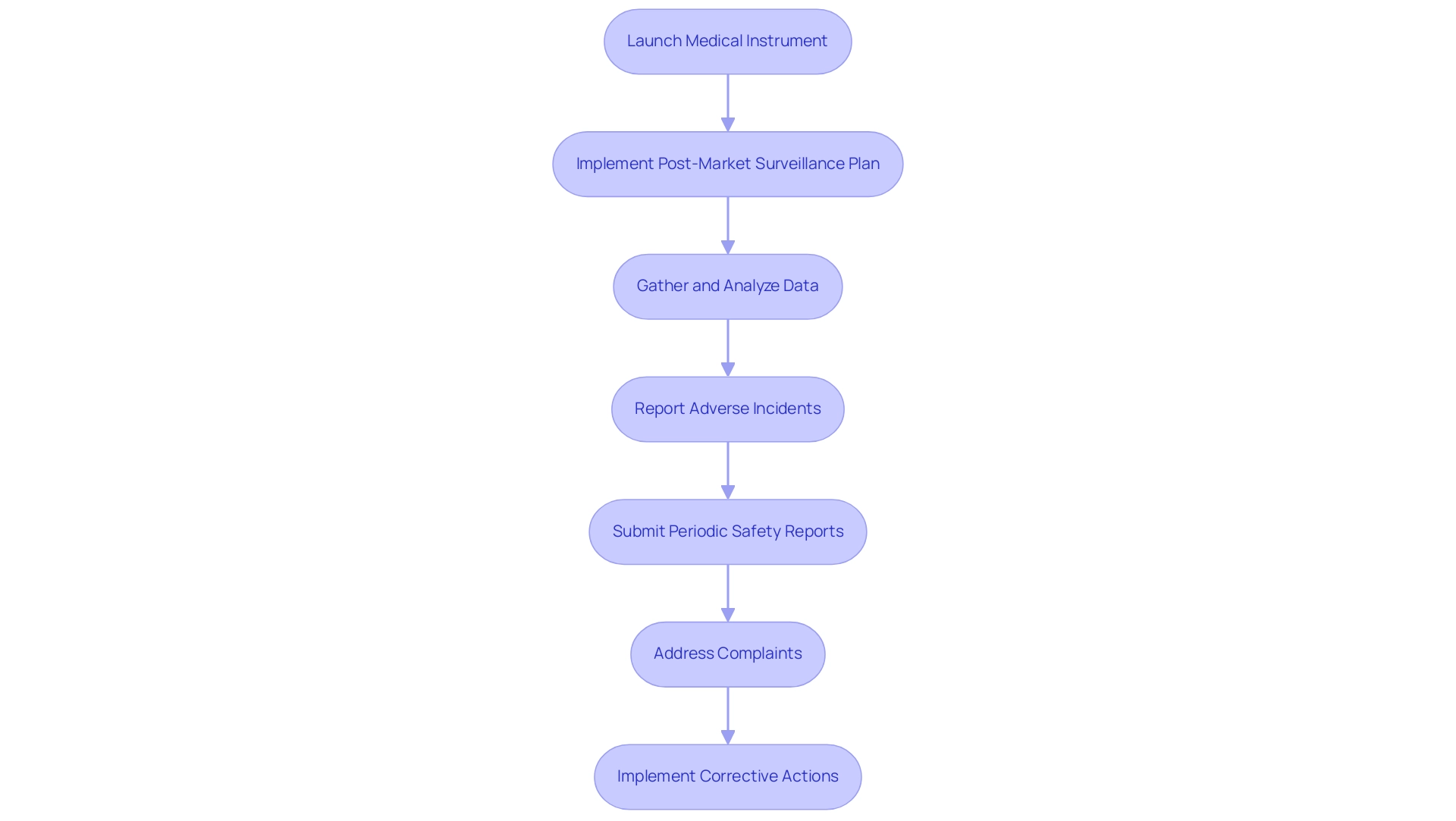
Once approval from authorities is secured and a medical instrument is launched, it is essential for manufacturers to implement a comprehensive post-market surveillance plan, as highlighted by experts like Katherine Ruiz and Ana Criado. Katherine, an industrial microbiologist with a Master's degree in Quality Management and Integrated Systems, has extensive experience advising foreign manufacturers on market clearance in Colombia. Ana, a biomedical engineering professor and an external regulatory consultant, has held leadership roles at INVIMA for over five years.
This plan should encompass systematic data gathering and analysis concerning the performance of the equipment, alongside any adverse incidents reported by users. In Brazil, the National Health Surveillance Agency (ANVISA) mandates that manufacturers submit periodic safety reports, emphasizing a proactive stance in monitoring the device's safety profile. It is crucial to reference Article 10 of RDC No. 67/2009, which outlines conditions exempting events from notification requirements, as this provides a clearer understanding of the oversight framework surrounding post-market surveillance.
Establishing effective channels for healthcare professionals and users to report issues is vital, as is ensuring a structured process for addressing complaints and implementing corrective actions where necessary. The urgency of these practices is underscored by the vigilance reporting guidelines for adverse events, which stipulate strict deadlines for reporting serious incidents, including fatalities or serious threats to public health.
For instance, manufacturers must report deaths within 24 hours and serious threats to public health within 72 hours. Adhering to these reporting timelines not only mitigates risks but also ensures compliance with health regulations, thereby safeguarding patient safety. As Margret Seidenfaden states, 'Choose the BRH carefully! He will hold your registration! You depend on him.' This highlights the importance of maintaining strong oversight relationships.
By prioritizing post-market surveillance, manufacturers not only enhance patient safety but also maintain their standing with oversight bodies, facilitated by the expertise of leaders like Katherine Ruiz and Ana Criado, thereby fostering ongoing trust in their products.
Engaging with Local Regulatory Experts and Consultants
Engaging local regulatory experts and consultants is essential for navigating regulatory requirements for medical devices in Latin America, specifically in Colombia, which stands out as a top destination for clinical trials. These professionals offer invaluable insights into local laws, regulations, and best practices crucial for the successful introduction of medical devices. Their expertise in preparing documentation, guiding through the IRB/EC approval procedures, and ensuring compliance with INVIMA, the Colombian National Food and Drug Surveillance Institute, is indispensable for navigating regulatory requirements for medical devices in Latin America.
Additionally, local consultants play a pivotal role in fostering relationships with governing entities and stakeholders, significantly enhancing the probability of successful market entry. With Colombia's healthcare system ranked among the best worldwide and its oversight methods being both efficient and rigorous, utilizing local expertise becomes even more essential. As highlighted by Julio G. Martinez-Clark, co-founder and CEO of bioaccess®, 'Colombia is a powerhouse in clinical research in Latin America.'
Furthermore, Colombia's membership in the OECD provides access to international best practices for clinical research, reinforcing the advantages of local consultancy. A case study on centralized regional administration of compliance affairs demonstrates how efficient methods can lead to reduced costs and enhanced market access. Additionally, the R&D tax incentives offered in Colombia—such as a 100% tax deduction on investments in science, technology, and innovation—further enhance the appeal for conducting clinical trials.
Therefore, conducting thorough research to identify reputable consultants with a proven track record in the medical device sector is crucial. Their specialized knowledge can lead to significant savings in both time and resources, ultimately facilitating a smoother pathway to market. Comprehensive clinical trial management services, including feasibility studies and compliance reviews, are also critical components in navigating regulatory requirements for medical devices in Latin America.
Conclusion
Successfully navigating the medical device regulatory landscape in Brazil and across Latin America necessitates a thorough understanding of the unique requirements established by local regulatory bodies. The Agência Nacional de Vigilância Sanitária (ANVISA) in Brazil, along with other authorities in the region, such as ANMAT in Argentina and COFEPRIS in Mexico, each present distinct challenges and opportunities that manufacturers must address. A clear grasp of:
- Device classification
- Comprehensive documentation requirements
- Adherence to Good Manufacturing Practices
is essential for regulatory approval and market entry.
Moreover, the importance of post-market surveillance cannot be overstated. Implementing a robust monitoring plan ensures that devices are continuously evaluated for safety and efficacy, aligning with regulatory mandates and fostering trust among healthcare professionals and patients alike. Engaging with local regulatory experts and consultants can significantly enhance compliance efforts, offering insights into region-specific nuances and facilitating smoother interactions with regulatory authorities.
In conclusion, as the medical device landscape evolves, staying informed and proactive is paramount. Embracing local expertise and maintaining rigorous compliance practices not only streamlines the path to market but also contributes to the overall safety and effectiveness of medical devices in Latin America. By prioritizing these strategies, manufacturers can position themselves for success in a complex and dynamic regulatory environment.
Frequently Asked Questions
What is the role of the Agência Nacional de Vigilância Sanitária (ANVISA) in Brazil's medical equipment approval framework?
ANVISA is the key authority overseeing the authorization of medical products in Brazil, responsible for ensuring compliance with regulatory standards.
How are medical instruments classified in Brazil?
Medical instruments in Brazil are categorized into four classes (I, II, III, and IV) based on their associated risk, with Class I representing the lowest risk and Class IV denoting the highest.
Which classes of medical products require registration with ANVISA?
Class III and IV products require registration with ANVISA.
How long is the authorization valid for registered medical products in Brazil?
The authorization for registered medical products is valid for ten years.
What documentation is needed for successful registration with ANVISA?
Successful registration requires the preparation of detailed technical files that include safety and efficacy data, which must be submitted to ANVISA for review.
Why is adherence to Brazilian Good Manufacturing Practices (BGMP) important?
Adherence to BGMP is critical because if a GMP certificate loses its validity, the device registration will also become invalid.
What ongoing responsibilities does ANVISA have regarding medical products?
ANVISA's oversight includes ongoing reviews of pharmacovigilance and CBD regulations, indicating a dynamic regulatory framework.
What services does bioaccess® provide for clinical trial management?
bioaccess® offers comprehensive clinical trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How can local specialists assist in navigating Brazil's regulatory landscape?
Partnering with local specialists, such as compliance consultants, can improve adherence to regulations and enhance the likelihood of successful market entry for healthcare products.
What should stakeholders be aware of when navigating regulatory requirements in Latin America?
Stakeholders should understand the specific requirements in each target country, as each has distinct procedures and documentation requirements, and they should stay informed about regional trends and policy updates to ensure compliance.




