Introduction
The classification of medical devices under the European Union Medical Device Regulation (EU MDR) is a critical component of ensuring safety and efficacy in healthcare. This structured methodology categorizes devices based on their intended use and associated risk levels, influencing everything from regulatory scrutiny to post-market surveillance obligations.
With the EU MDR delineating three primary categories—Class I, Class II, and Class III—manufacturers, regulatory authorities, and healthcare professionals must navigate a complex framework that directly impacts market access and compliance.
As the landscape evolves, particularly with the impending deadlines for compliance and the heightened scrutiny of high-risk devices, understanding the nuances of this classification system becomes essential for all stakeholders involved in the medical device lifecycle.
Understanding Medical Device Classification Under EU MDR
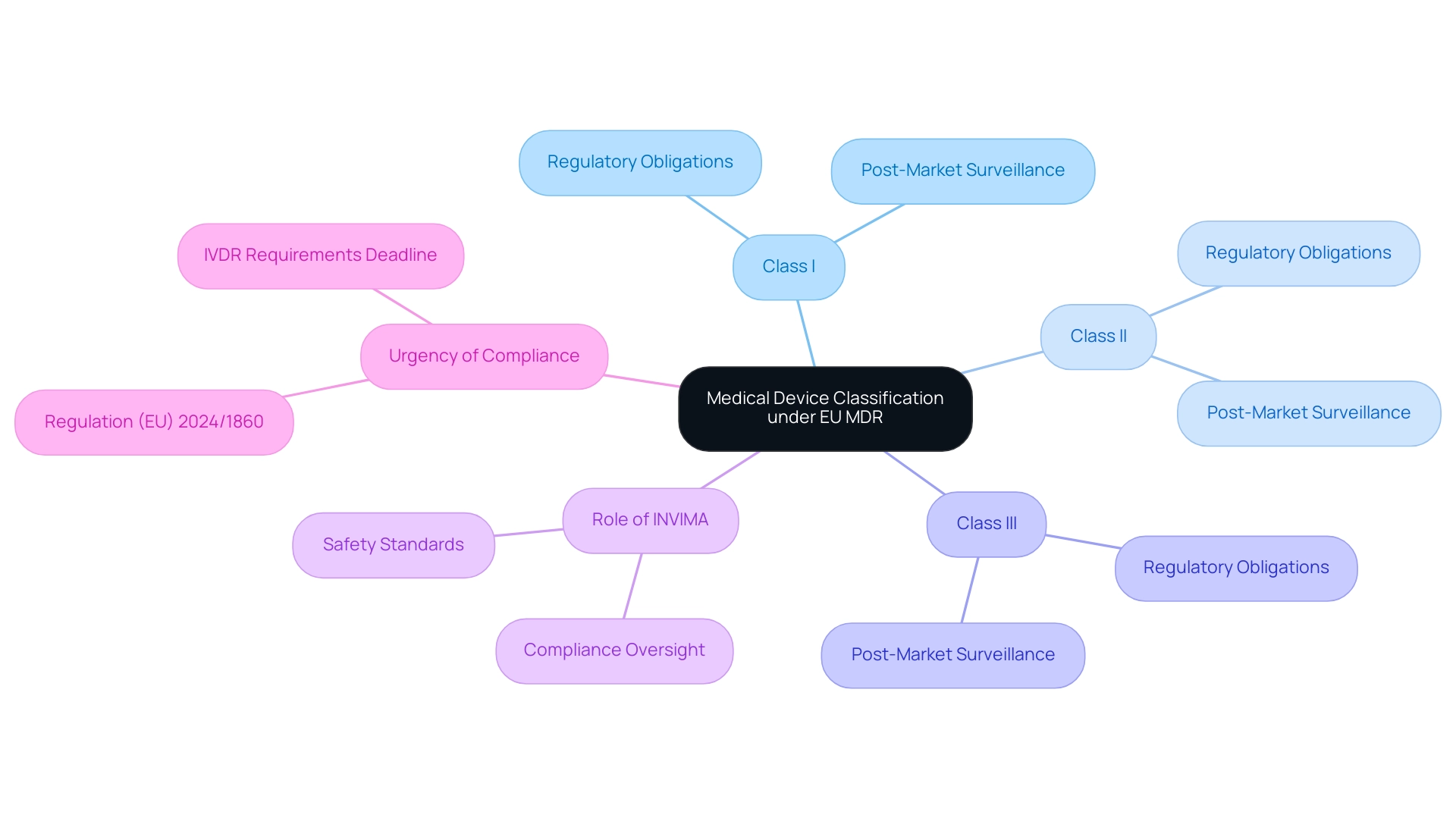
The medical device classification MDR under the European Union Medical Device Regulation (EU MDR) represents a structured methodology that categorizes healthcare instruments according to their intended use and associated risk levels. This categorization system is vital for ensuring that medical products comply with strict safety and efficacy criteria before entering the market. The EU MDR outlines a framework for medical device classification mdr that categorizes products into three primary groups:
- Class I
- Class II
- Class III
Each group has specific regulatory obligations.
The medical device classification mdr not only determines the intensity of scrutiny a product faces during its approval process but also significantly affects post-market surveillance and compliance responsibilities. In Colombia, the regulation of healthcare instruments falls under the jurisdiction of INVIMA (Colombia National Food and Drug Surveillance Institute), which is responsible for inspecting and supervising the marketing and production of health products. The Directorate for Healthcare Instruments and other Technologies plays a crucial role within INVIMA, monitoring and controlling healthcare tools, suggesting technical standards, and ensuring compliance with pre- and post-market requirements.
As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that healthcare products meet high safety, efficacy, and quality standards. Navigating the medical device classification mdr is essential for manufacturers, regulatory bodies, and healthcare experts, as it directly impacts the development, approval, and market access of healthcare products. As Saint-Gobain states, 'We’re here to help navigate, contact us if you have questions,' emphasizing the importance of support in this complex landscape.
Moreover, the recent case study concerning Regulation (EU) 2024/1860 emphasizes the urgency for manufacturers to adhere to IVDR requirements by the deadline of 26 May 2025, especially considering potential public health risks due to shortages of in vitro diagnostic products. This situation underscores INVIMA's role in ensuring compliance and safety for medical devices in the Colombian market. Furthermore, with four applications chosen as test cases for the first phase of the pilot, this statistic illustrates the sorting process in action.
As new regulations unfold, awareness of the medical device classification mdr is crucial for maintaining compliance and ensuring patient safety.
Exploring Classifications: Class I, II, and III Medical Devices
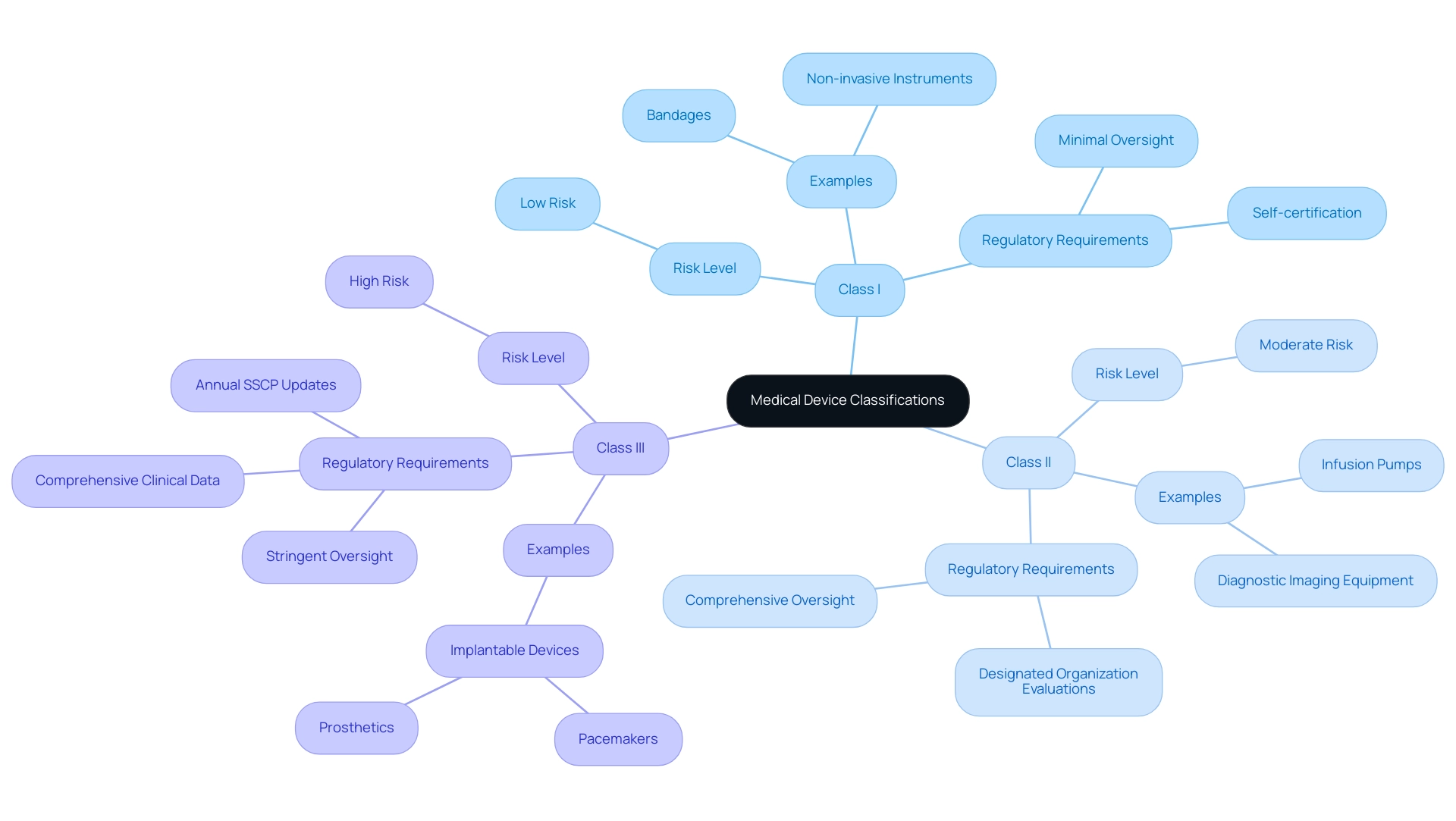
The EU Medical Equipment Regulation (MDR) categorizes medical instruments into three distinct classes: Class I, Class II, and Class III. Class I products, deemed low risk, typically include everyday items like bandages and non-invasive instruments, requiring minimal regulatory oversight. Producers of these products often appreciate the ability to self-certify adherence to applicable regulations.
In contrast, Class II items, which present a moderate risk, cover a wider array of products, such as infusion pumps and diagnostic imaging equipment. These instruments require a more comprehensive oversight assessment, including evaluations by designated organizations to ensure safety and effectiveness. Class III products signify the highest risk category, including implantable items such as pacemakers and prosthetics, subjected to the most stringent oversight demanding comprehensive clinical data and continuous post-market clinical follow-up (PMCF) to confirm safety and performance.
As noted by Laura Court, 'A successful clinical evaluation report hinges on the quality of clinical data and the processes for collecting and analyzing it.' Furthermore, the importance of ongoing updates is illustrated by the Summary of Safety and Clinical Performance (SSCP), which must be updated annually for implantable Class III devices, incorporating findings from PMCF and PSUR. This governance framework is crucial for ensuring patient safety and enhancing healthcare efficiency, as evidenced by the statistic that 83.3% of large enterprises believe health technology reduces healthcare costs.
A comprehensive grasp of these categories, along with bioaccess®'s expertise in handling Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, is crucial for manufacturers to maneuver the intricate compliance framework and ensure adherence to the medical device classification MDR. Bioaccess® offers extensive services, including feasibility studies, site selection, and compliance reviews, all directed by the expertise of Katherine Ruiz in Regulatory Affairs for healthcare products and in vitro diagnostics in Colombia.
Determining Risk Classification for Medical Devices
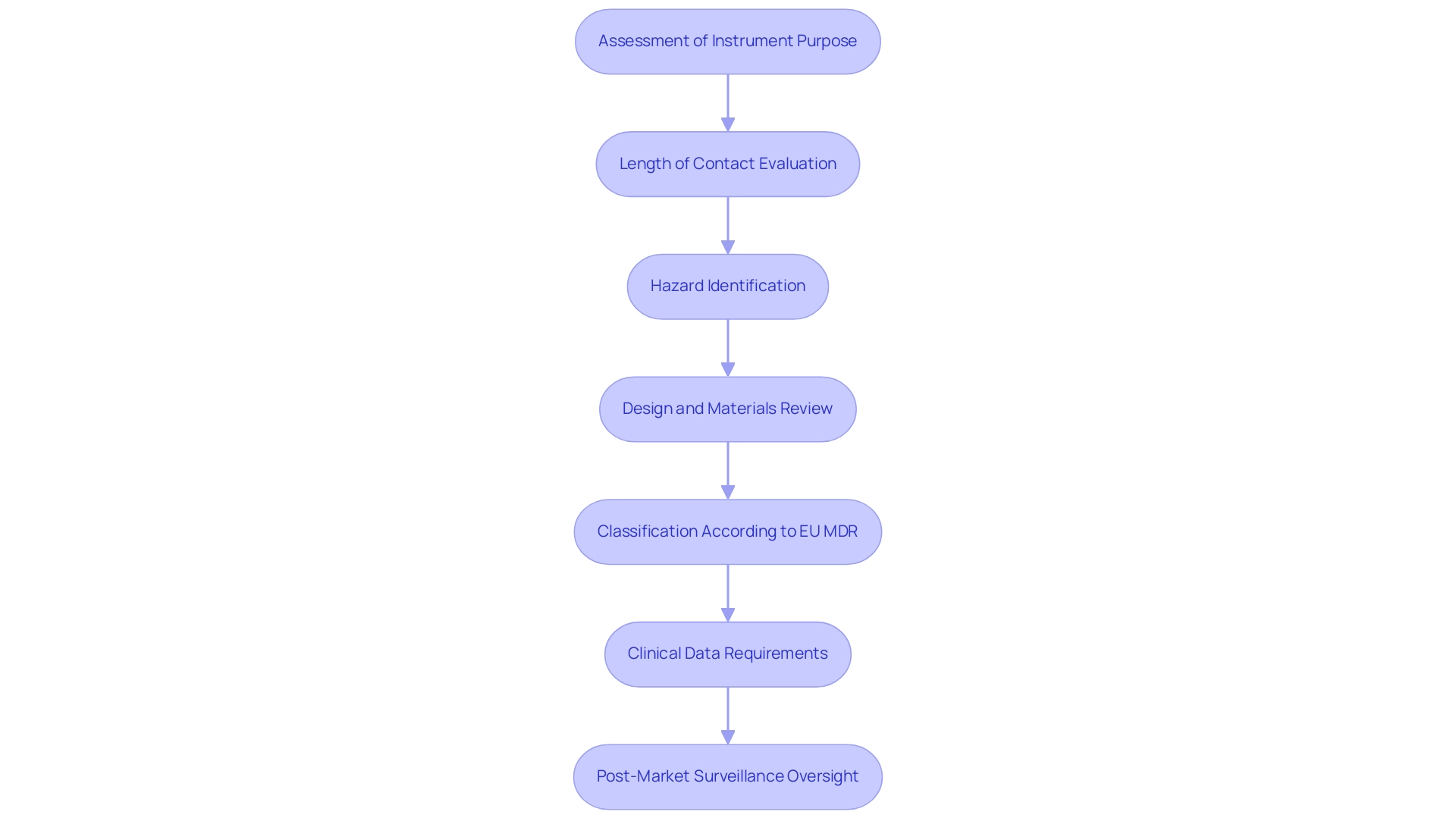
Establishing the risk categorization for medical instruments requires a comprehensive assessment of various elements, such as the instrument's intended purpose, length of contact with the body, and the related potential hazards. Manufacturers are tasked with assessing aspects such as design, materials, and mode of action to accurately determine categorization. According to the EU MDR, specific criteria and guidelines are available to support manufacturers through the medical device classification MDR process, particularly the categorization rules detailed in Annex VIII of the regulation, which outline the criteria for determining whether an item falls into Class I, IIa, IIb, or III based on its risk profile and intended use.
For instance, instruments intended for invasive procedures generally fall into higher risk categories compared to non-invasive counterparts. This categorization under the medical device classification MDR is not merely procedural; it significantly influences the regulatory pathway, dictating the type of clinical data required for approval and the extent of oversight necessary during post-market surveillance. As emphasized by Murugan Kandasamy, Managing Director & CEO at DQS India, 'Nonetheless, maneuvering through the categorization route can be difficult, especially for intricate or novel healthcare instruments.'
This emphasizes the critical nature of the risk categorization process, which ultimately aims to enhance patient safety and ensure compliance with current regulations. Furthermore, in 2023, certificated Class III health products constituted 11% of items recorded in EUDAMED, illustrating the ongoing importance of rigorous classification standards in the healthcare landscape. A crucial document in this context is the Clinical Evaluation Report (CER), which serves as a mandatory component for all healthcare products sold in the EU, detailing the clinical evaluation process essential for demonstrating safety and performance.
The CER must be comprehensive and cross-referenced with supporting documents to ensure compliance with EU regulations. Furthermore, healthcare equipment producers must follow specific labeling standards outlined in the medical device classification MDR to ensure safe and effective use, which further underscores the significance of adherence within the compliance framework. Our comprehensive clinical trial management services support manufacturers through:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
By integrating these services, we enhance the clinical evaluation process, ensuring that products meet the necessary safety and performance criteria while navigating the complexities of risk classification and regulatory compliance.
Four Steps to Classify a Medical Device Under EU MDR
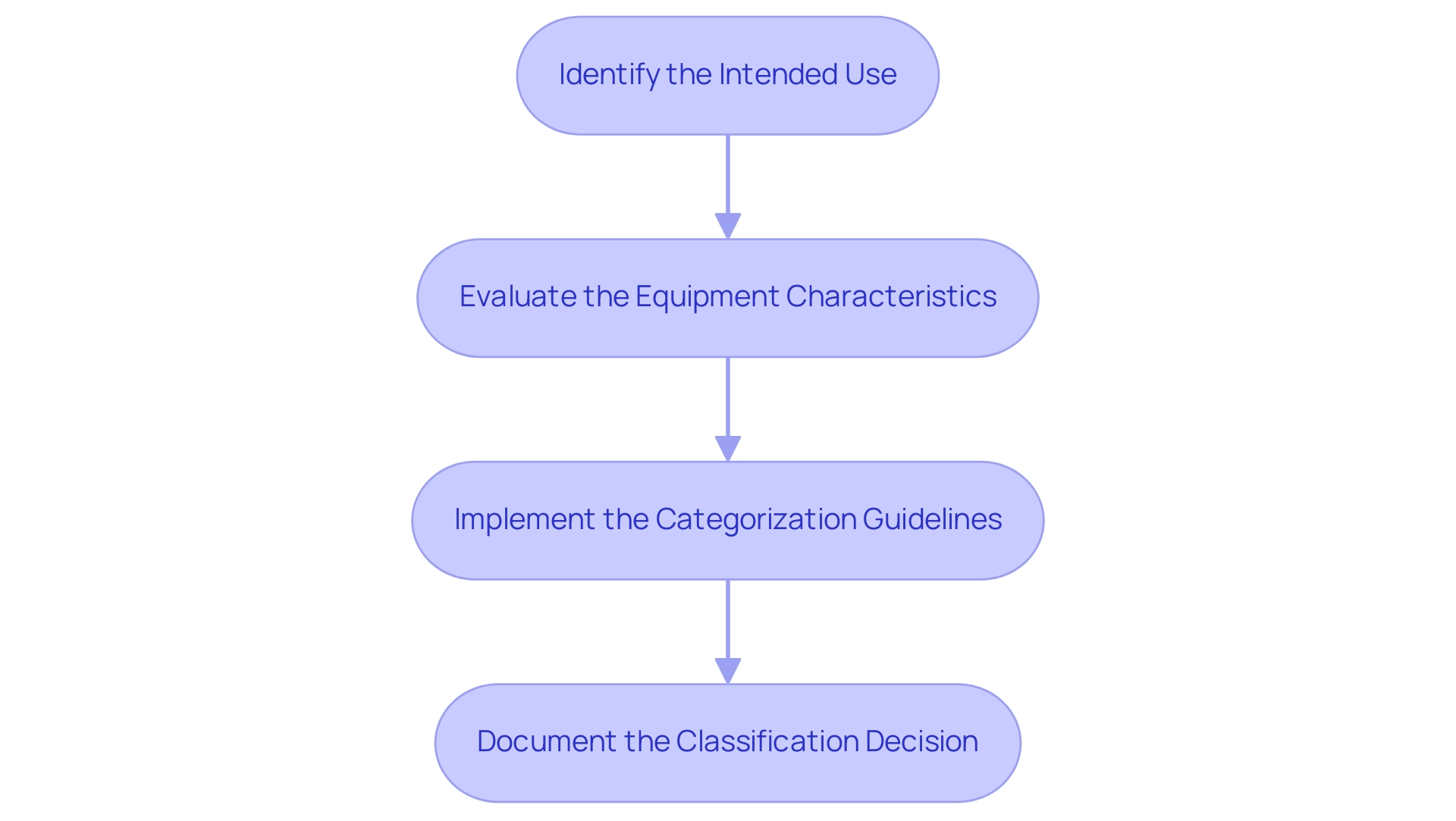
The medical device classification MDR involves a structured process for classifying a healthcare instrument under the EU Medical Equipment Regulation, consisting of four essential steps designed to ensure compliance and facilitate market access. The EU MDR document itself is extensive, comprising 10 Chapters, 123 Articles, 17 Annexes, and a total of 175 pages, reflecting the complexity of the regulations governing medical equipment. These steps are as follows:
- Identify the Intended Use: The first step involves a precise definition of the item's purpose and the specific patient population it targets. This clarity is critical as it informs subsequent evaluations and regulatory requirements.
- Evaluate the Equipment Characteristics: Manufacturers must assess the design, materials, and technological features of the equipment to establish its risk profile. This evaluation is vital to understanding how the instrument may interact with patients and the environment.
- Implement the Categorization Guidelines: The categorization guidelines specified in Annex VIII of the EU must be utilized to ascertain the suitable class for the item based on its intended use and features according to the medical device classification MDR. Grasping the categorization is crucial, as it determines the compliance standards that need to be fulfilled. As Bruna De Lucca Caetano rightly points out, understanding the categorization of a healthcare instrument is crucial since it determines the compliance standards that such instruments must meet. Notably, within Class I, there are three sub-classifications that require notified body involvement: Class Is (sterile), Class Im (measuring feature), and Class Ir (reusable surgical instrument).
- Document the Classification Decision: Comprehensive documentation is crucial in this step. Manufacturers should keep detailed records of their sorting process, including justifications for the chosen categorization. This documentation not only aids in compliance with standards but also equips the manufacturer for possible evaluations.
Ana Criado, our Director of Compliance Affairs and a seasoned expert in the field, emphasizes the significance of these steps in ensuring that medical products meet the medical device classification MDR and fulfill the necessary requirements for market entry while promoting safety and efficacy. Drawing on her extensive experience at INVIMA, where she navigated complex regulatory frameworks, Ana highlights that a thorough understanding of the classification process is essential for compliance and effective market entry. Additionally, the Post-market Clinical Follow-up (PMCF) plan is a formal requirement under the EU MDR, which Ana emphasizes is crucial for ongoing data collection and assessment to ensure safety and efficacy. Her insights highlight the significance of systematic documentation practices, especially as deadlines for compliance near, such as the requirement for Class III custom-made implantable items to comply by May 26, 2026.
Navigating the Implications of MDR Classification for Manufacturers
The medical device classification mdr of healthcare products under the EU MDR carries significant implications for producers, dictating specific compliance requirements for approval, post-market monitoring, and quality management systems (QMS). Our comprehensive clinical trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that all aspects of the process are meticulously handled. Specifically, we provide review and feedback on study documents to comply with country requirements and detailed reporting on study status, inventory, and both serious and non-serious adverse events.
Notably, 69% of participants in a recent analysis were categorized into the 'High-Risk Device Group', underscoring the heightened scrutiny faced by these manufacturers. For instance, while Class I products may face fewer compliance challenges, those involved in the medical device classification mdr for Class III items must manage extensive clinical trials and continuous oversight, which can be resource-intensive. Katherine Ruiz, a specialist in compliance matters for medical instruments and in vitro diagnostics in Colombia, highlights the significance of having the appropriate tools established for QMS and documentation, stating, 'Having the right tools in place for QMS and documentation is a crucial success factor for the time to product marketing and thus for the business success of your company.'
Furthermore, a recent survey revealed that 83.3% of large enterprises believe the MDR guarantees fair market access, highlighting the perceived benefits despite the challenges. Manufacturers must remain vigilant regarding updates to the EU MDR related to medical device classification mdr, as failure to comply can result in severe penalties, including market withdrawal. Recent amendments from the E.U. Health Commissioner indicate a recognition of these challenges, allowing more time for manufacturers and notified bodies to certify devices amidst concerns about supply shortages. The re-certification of existing products has emerged as a significant challenge, compelling manufacturers to undertake extensive documentation updates and follow stricter compliance standards. This process often requires systematic reviews of existing products against the medical device classification MDR requirements and early engagement with Notified Bodies to streamline re-certification procedures.
Understanding these implications enables manufacturers to strategically navigate the regulatory landscape, ensuring successful market entry and sustained compliance.
Conclusion
The classification of medical devices under the EU Medical Device Regulation (MDR) is a pivotal aspect of ensuring safety, efficacy, and compliance within the healthcare sector. By categorizing devices into Classes I, II, and III based on their intended use and associated risks, the regulation establishes a clear framework that guides manufacturers through the complexities of market access and regulatory obligations. Each class presents distinct challenges and requirements, highlighting the critical nature of understanding the nuances involved in the classification process.
As the landscape of medical device regulation evolves, particularly with impending deadlines for compliance, stakeholders—including manufacturers, regulatory authorities, and healthcare professionals—must prioritize their understanding of these classifications. The implications of classification extend beyond initial approval; they encompass ongoing post-market surveillance, quality management systems, and compliance with stringent documentation practices. This comprehensive awareness is essential not only for meeting regulatory expectations but also for ensuring patient safety and maintaining public trust in medical technologies.
In conclusion, navigating the intricacies of the EU MDR classification system is essential for all parties involved in the medical device lifecycle. By adhering to the established guidelines and remaining proactive in compliance efforts, stakeholders can effectively mitigate risks, enhance product safety, and ultimately contribute to improved healthcare outcomes. The commitment to understanding and applying these regulations will foster an environment of innovation and reliability in the medical device market, ensuring that high standards are upheld for the benefit of patients and healthcare providers alike.
Frequently Asked Questions
What is the purpose of the medical device classification under the EU MDR?
The medical device classification under the EU MDR categorizes healthcare instruments according to their intended use and associated risk levels, ensuring compliance with strict safety and efficacy criteria before products enter the market.
What are the three primary classes of medical devices according to the EU MDR?
The three primary classes of medical devices are Class I, Class II, and Class III, each with specific regulatory obligations.
How does the classification of a medical device affect its approval process?
The classification determines the intensity of scrutiny a product faces during its approval process and significantly affects post-market surveillance and compliance responsibilities.
What role does INVIMA play in the regulation of healthcare instruments in Colombia?
INVIMA, the Colombia National Food and Drug Surveillance Institute, is responsible for inspecting and supervising the marketing and production of health products, ensuring that they meet high safety, efficacy, and quality standards.
What are the characteristics of Class I medical devices?
Class I medical devices are deemed low risk and include everyday items like bandages and non-invasive instruments, requiring minimal regulatory oversight and allowing producers to self-certify compliance.
What types of products fall under Class II medical devices?
Class II medical devices present a moderate risk and include products such as infusion pumps and diagnostic imaging equipment, requiring more comprehensive oversight and evaluations by designated organizations.
What distinguishes Class III medical devices from the other classes?
Class III medical devices signify the highest risk category, including implantable items like pacemakers and prosthetics, and are subjected to the most stringent oversight, requiring comprehensive clinical data and continuous post-market clinical follow-up.
What is the importance of the Summary of Safety and Clinical Performance (SSCP)?
The SSCP must be updated annually for implantable Class III devices, incorporating findings from post-market clinical follow-up and ensuring ongoing compliance with safety and performance standards.
How can manufacturers navigate the medical device classification framework?
Manufacturers can navigate the framework by understanding the classifications, utilizing services for feasibility studies, site selection, and compliance reviews, and leveraging expertise in regulatory affairs.
What is the deadline for manufacturers to adhere to IVDR requirements as per Regulation (EU) 2024/1860?
Manufacturers must adhere to IVDR requirements by the deadline of 26 May 2025, highlighting the urgency for compliance due to potential public health risks.




