Overview
This article serves as a comprehensive guide for navigating the INVIMA clinical trial submission process in Colombia. It outlines essential documentation, key regulations, and detailed step-by-step procedures necessary for successful approval. The importance of thorough preparation and strict compliance with INVIMA's requirements is emphasized, alongside insights into common challenges faced during the process. Additionally, the article highlights the economic advantages of conducting research in Colombia, effectively positioning the country as an attractive destination for clinical trials.
Introduction
In the dynamic landscape of clinical research, understanding the regulatory framework is paramount, particularly in Colombia, where the National Food and Drug Surveillance Institute (INVIMA) serves as the cornerstone of medical product oversight.
As the primary authority responsible for ensuring the safety and efficacy of clinical trials, INVIMA plays a pivotal role in shaping the research environment. Researchers must navigate a complex array of regulations, documentation requirements, and compliance obligations to achieve successful trial approval.
With Colombia emerging as a cost-effective destination for clinical studies, driven by INVIMA's proactive initiatives, a comprehensive grasp of the submission process can significantly enhance the likelihood of success.
This article delves into INVIMA's essential functions, the critical documentation required for submissions, and the key regulations that govern clinical trials, providing researchers with the insights needed to thrive in this competitive arena.
Understanding INVIMA's Role in Clinical Trials in Colombia
The National Food and Drug Surveillance Institute serves as Colombia's principal regulatory authority, tasked with ensuring the safety, effectiveness, and quality of medical products, including those associated with research studies. This regulatory body is crucial in evaluating research study applications, supervising ongoing investigations, and guaranteeing compliance with both national and international regulations. A comprehensive grasp of the agency's functions is vital for researchers aiming to align their submissions with regulatory expectations, thus facilitating a more streamlined INVIMA clinical trial submission process.
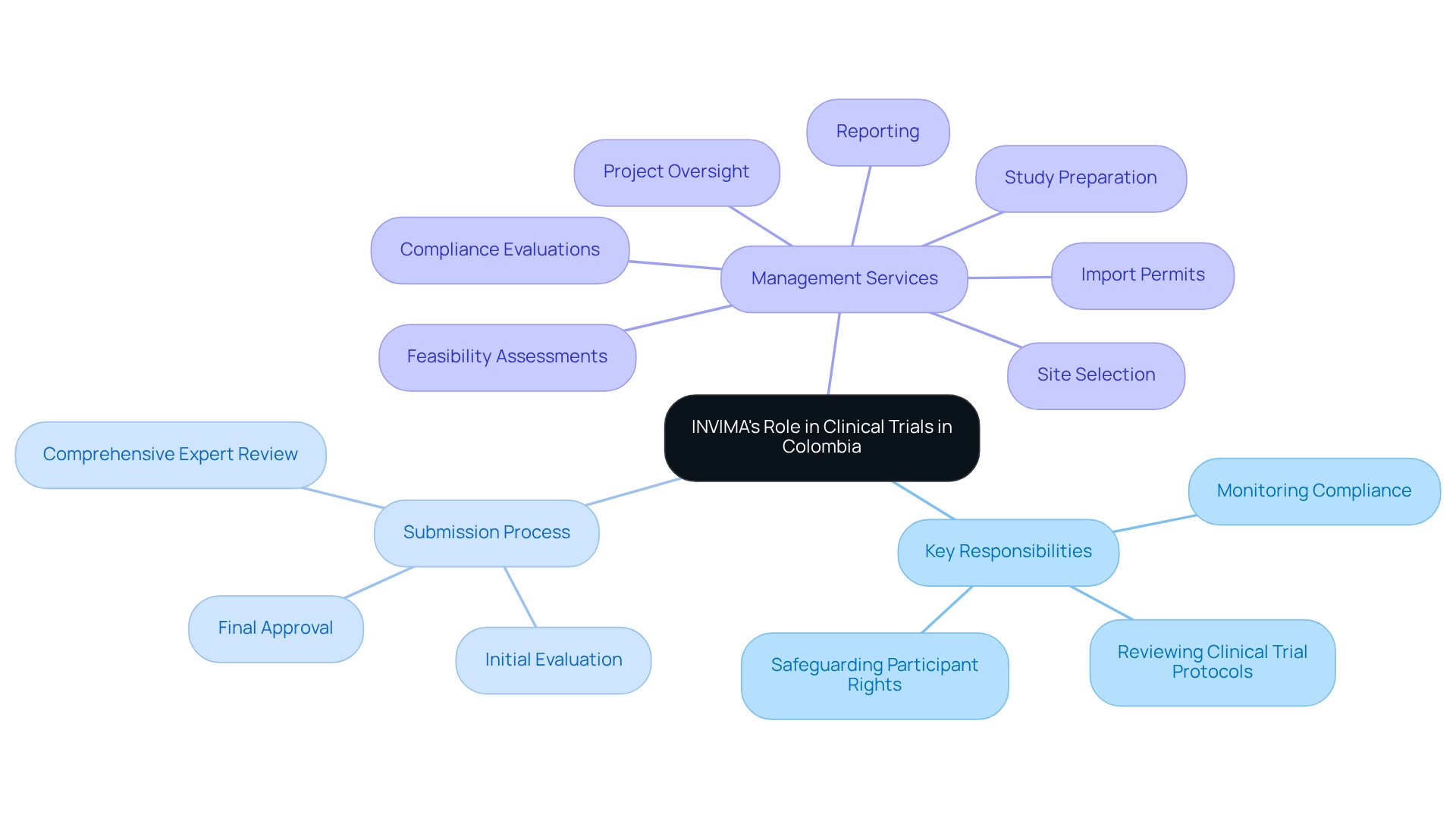
Key responsibilities of INVIMA include:
- Reviewing Clinical Trial Protocols: INVIMA meticulously evaluates clinical trial protocols to ensure adherence to ethical and scientific standards, which is essential for maintaining the integrity of the research.
- Monitoring Compliance: The institute ensures that all research studies comply with Good Clinical Practice (GCP) guidelines, safeguarding the quality of the investigations conducted.
- Safeguarding Participant Rights: The regulatory body is dedicated to upholding the rights and well-being of research participants, a fundamental aspect of ethical medical studies.
Recent updates indicate that the assessment method for research study applications aligns with the INVIMA clinical trial submission process, which involves an initial evaluation, a comprehensive expert review, and final approval by a higher-level committee. This rigorous procedure underscores the organization’s commitment to maintaining high standards in medical research.
In addition to INVIMA's regulatory functions, bioaccess offers extensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, study preparation, import permits, nationalization of investigational devices, project oversight, and reporting on study status, inventory, and adverse events. This holistic approach ensures that all aspects of the research process are effectively managed, allowing investigators to focus on their primary objectives.
Colombia's cost-effective environment for medical studies further enhances the appeal of conducting research in the country. Medical procedures can be 40% to 75% less expensive than in the U.S., enabling more extensive studies without sacrificing quality. This economic advantage, coupled with a proactive regulatory framework, positions Colombia as an attractive destination for medtech companies looking to conduct research.
As Julio G. Martinez-Clark, CEO, noted, 'The government of Colombia appears to be the only nation in Latin America with a proactive effort to draw more research studies as part of its strategy to evolve into a knowledge economy by 2031.'
Understanding the role and responsibilities of the regulatory body, along with the comprehensive services provided by bioaccess, is the first step toward effectively navigating the INVIMA clinical trial submission process in Colombia, ultimately contributing to the advancement of medical knowledge and patient care.
Essential Documentation for INVIMA Submission
Submitting a research study application to the regulatory authority necessitates meticulous preparation of various essential documents, each playing a pivotal role in the approval process. Bioaccess offers comprehensive clinical study management services designed to facilitate the INVIMA clinical trial submission process, ensuring compliance with INVIMA regulations.
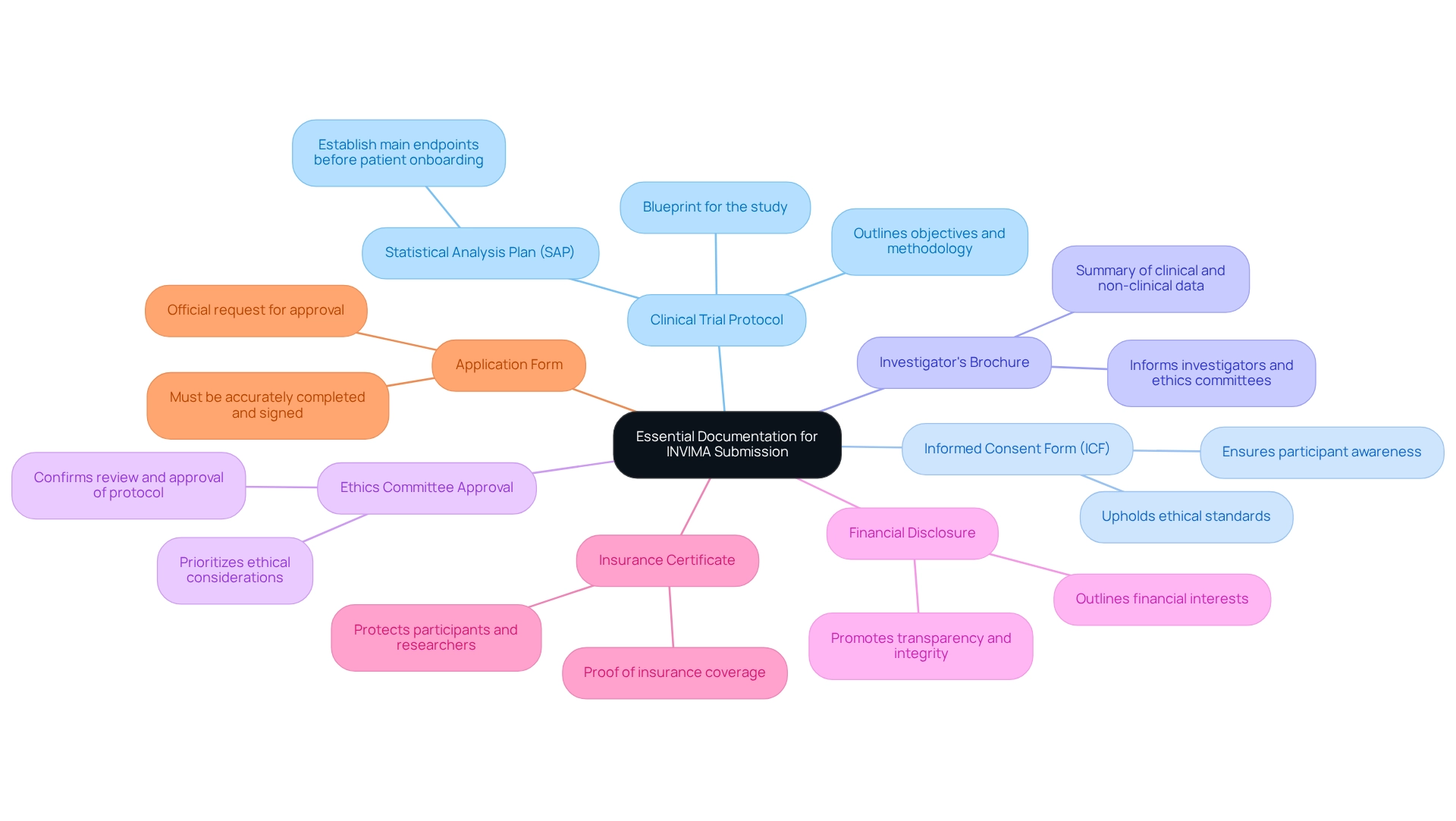
- Clinical Trial Protocol: This document acts as a blueprint for the study, outlining its objectives, methodology, and statistical analysis plan (SAP). It is critical that the main endpoints are established in the SAP before the onboarding of the first patient, ensuring clarity and focus throughout the study.
- Informed Consent Form (ICF): This document guarantees that participants are fully informed about the study's nature, risks, and benefits, and that they voluntarily agree to participate. Clear communication within the ICF is vital to uphold ethical standards.
- Investigator's Brochure: A thorough summary of both clinical and non-clinical data regarding the investigational product, this document is essential for informing investigators and ethics committees about the product's safety and efficacy.
- Ethics Committee Approval: A letter from the local ethics committee is required to confirm that the study protocol has undergone review and approval, ensuring that ethical considerations are prioritized.
- Financial Disclosure: This document outlines any financial interests or conflicts of interest of the researchers involved in the study, promoting transparency and integrity in the research process.
- Insurance Certificate: Proof of insurance coverage for study participants is necessary to protect both the participants and the researchers from potential liabilities.
- Application Form: The official application form from the regulatory authority must be accurately completed and signed, serving as the formal request for approval of the experiment.
Accurate and comprehensive completion of these documents is imperative for the success of the INVIMA clinical trial submission process. Common errors in documentation can result in delays or rejections, underscoring the necessity of thoroughness. For instance, a recent study entitled 'Guidelines for Statistical Analysis Plans in Clinical Trials' highlighted the need for transparency in statistical analysis plans, recommending a minimum collection of 63 vital items to enhance the integrity of study execution and reporting.
As Carrol Gamble, PhD, observed, this guidance assumes that the SAP is not a standalone document; thus, it is unnecessary to replicate extensive portions of the protocol, which should be clearly referenced instead. By adhering to these guidelines and ensuring that all documentation is meticulously prepared, researchers can significantly improve their chances of a successful application for approval. Moreover, transforming experimental designs into actionable strategies is crucial for seamless delivery and successful outcomes.
Key INVIMA Regulations Impacting Clinical Trials
In Colombia, several crucial regulations established by INVIMA govern the research study environment, a vital component of the INVIMA clinical trial submission process, ensuring both ethical and scientific integrity. Among these, Resolution 2378 of 2014 stands out as particularly significant, as it delineates the requirements for conducting medical studies, including the necessity for ethical approval and strict adherence to Good Clinical Practice (GCP). This regulation has played a pivotal role in shaping the research landscape, fostering a culture of compliance and ethical responsibility.
Another essential regulation is Resolution 8430 of 1993, which provides comprehensive guidelines for the registration of research trials. It outlines the responsibilities of sponsors and researchers, ensuring that all participants are fully aware of their commitments and the standards expected in medical research.
Moreover, Decree 1787 of 2014 establishes a robust framework for the protection of human subjects involved in medical research. This decree underscores the importance of informed consent and the rights of participants, which are critical for upholding ethical standards in research studies.
Understanding these regulations is imperative for researchers aiming to navigate the research study environment in Colombia effectively. With Colombia ranking fourth in Latin America for recruiting and not-yet recruiting studies per million people, at a rate of 4.65, the regulatory environment promotes a cost-effective approach to conducting extensive studies. This not only benefits patients but also significantly advances medical knowledge.
As Julio G. Martinez-Clark, CEO of bioaccess Colombia, notes, "Colombia’s mixture of a large and diverse population, seasoned research sites, and effective regulatory processes make it a compelling option for U.S. medical device firms."
Additionally, bioaccess offers a wide array of research management services, including feasibility studies, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. This comprehensive approach ensures that all aspects of research studies are meticulously managed in accordance with regulatory standards, facilitating compliance and enhancing the success of trials.
The cultural context in which these regulations operate is also significant. For example, a case study on cultural sensitivity in informed consent illustrates how cultural differences can impact patient perceptions of medical recommendations. This highlights the necessity of addressing these differences while respecting local traditions to protect patient rights and ensure ethical adherence in research, particularly regarding the informed consent requirements established by regulations.
As of 2025, remaining informed about the INVIMA clinical trial submission process and its implications is essential for researchers in Colombia, especially in light of ongoing developments and updates to INVIMA's regulatory framework. This knowledge will facilitate a smoother approval process and enhance the overall success of medical studies in the region, supported by the expertise of professionals like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia.
Step-by-Step Guide to the Clinical Trial Approval Process
The medical study approval process in Colombia is organized and encompasses several essential steps that must be carefully adhered to in order to guarantee prompt approval.
Preparation of Documentation: Begin by gathering all essential documents, including the clinical trial protocol, informed consent forms, and approvals from the ethics committee. Thorough preparation is crucial, as incomplete documentation can lead to delays.
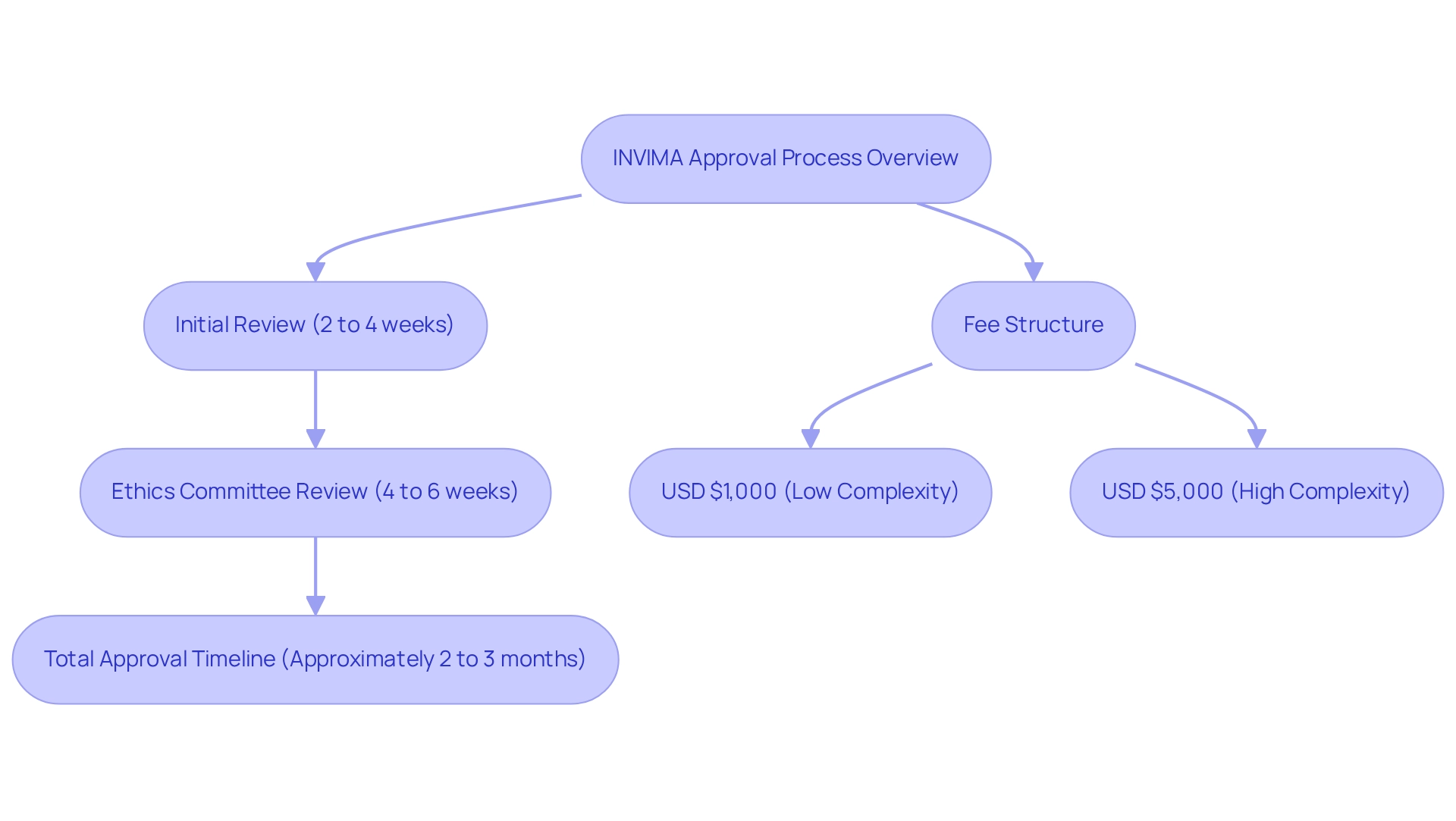
As part of the INVIMA clinical trial submission process, submit the complete application package to the regulatory agency, ensuring that all forms are accurately filled out. This initial submission is a crucial step in the procedure, as the national authority responsible for medical device regulation in Colombia is classified as a Level 4 health authority by PAHO/WHO. INVIMA will conduct an initial review of the submitted documents, which typically spans 2 to 4 weeks. During this period, it is vital to remain accessible for any clarifications that may be required.
Ethics Committee Review: Concurrently, the local ethics committee will evaluate the study protocol, a procedure that may require an additional 4 to 6 weeks. This dual evaluation method is designed to ensure that all ethical considerations are addressed, reflecting the commitment to patient safety and regulatory compliance.
Response to Queries: Be prepared to reply quickly to any questions or requests for further information from the regulatory agency. Effective communication can significantly expedite the review process, showcasing the importance of collaboration between sponsors and regulatory bodies.
Approval Notification: After completing both evaluations, the agency will send an approval notice, indicating that the study can begin. This notification is a crucial milestone in the research journey, signifying the shift from planning to execution.
Study Initiation: Once authorization is granted, commence the research according to the approved protocol, ensuring continuous adherence to the INVIMA clinical trial submission process throughout the study.
Following these steps can significantly improve the chances of a successful approval. Notably, the median duration for regulatory approval in South America has been reported at 236 days, emphasizing the importance of efficient methods and proactive management of timelines. Furthermore, operational methods, like those adopted at the Mayo Clinic, have shown that simultaneous activation steps can decrease activation duration by as much as 70%, highlighting the potential for efficiency in research processes.
By adopting this organized method and utilizing the knowledge of partners like bioaccess®, which provides extensive services such as feasibility studies, site selection, compliance assessments, and project management, organizations can effectively manage the intricacies of regulatory requirements. Moreover, with the guidance of experts like Katherine Ruiz in Regulatory Affairs, sponsors can ensure a smoother path to market access in the Latin American Medtech landscape.
Post-Approval Compliance and Reporting Obligations
Upon receiving approval from INVIMA, researchers are mandated to fulfill several critical compliance and reporting obligations to ensure the integrity and safety of clinical trials. Regular reporting is essential; researchers must submit periodic reports to INVIMA detailing the clinical trial's progress. These reports should encompass information on any adverse events or protocol deviations, which are crucial for maintaining transparency and participant safety. Additionally, it is imperative to swiftly report any serious adverse events to both the regulatory authority and the ethics committee. This prompt communication prioritizes participant safety and addresses any potential hazards related to the study.
Upon completion of the study, a comprehensive final report must be submitted as part of the INVIMA clinical trial submission process. This report should summarize the study's findings, outcomes, and any significant insights gained during the research process. Compliance with Good Clinical Practice (GCP) throughout the study is essential, as it guarantees the reliability of the data gathered and upholds the ethical standards of medical research.
Upholding these compliance requirements is vital not only for the successful execution of research studies but also significantly contributes to establishing trust with regulatory bodies. In Colombia, statistics reveal that 19.3% of individuals in research studies did not complete follow-up surveys. This underscores the importance of robust reporting systems to enhance participant involvement and adherence. As Goldacre noted, "In the absence of meaningful work from the FDA to police compliance, the only thing left is public accountability," emphasizing the critical nature of compliance and reporting obligations.
Moreover, a case study titled 'Role of Compliance in Web-Based Cognitive Behavioral Therapy Outcomes' demonstrates that effective compliance strategies can substantially reduce risks and enhance study results, further emphasizing the importance of meticulous adherence to regulatory requirements.
At bioaccess, we specialize in extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
We ensure that all elements of the research study comply with the INVIMA clinical trial submission process and its rigorous regulations.
Common Challenges in the INVIMA Submission Process and How to Overcome Them
Researchers frequently face numerous challenges during the INVIMA clinical trial submission process, which can significantly influence both the timeline and the success of clinical trials. Key obstacles include:
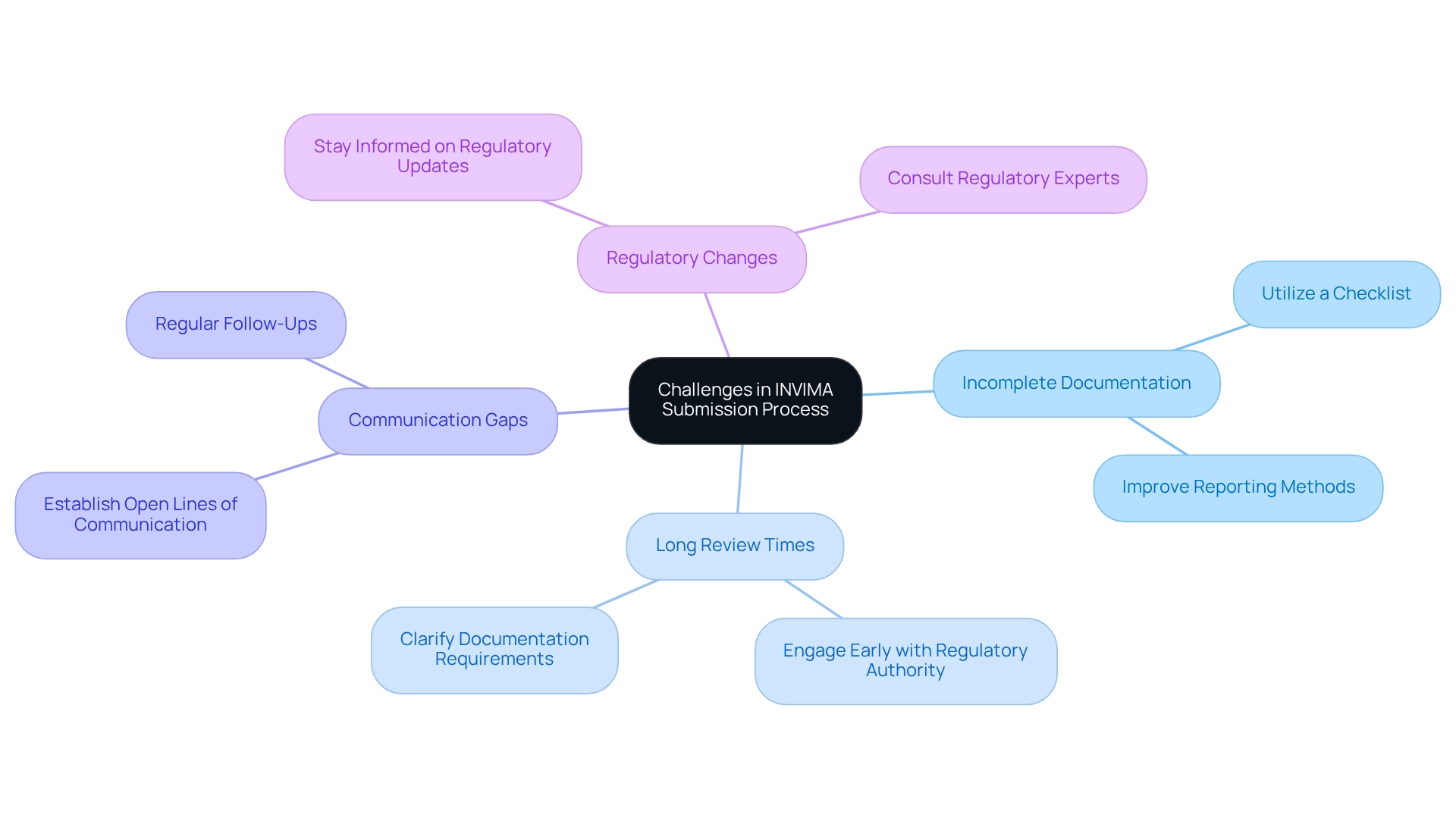
- Incomplete Documentation: A prevalent issue is the submission of incomplete or inaccurately filled documents. To mitigate this risk, it is crucial to conduct a meticulous review utilizing a comprehensive checklist that aligns with the INVIMA clinical trial submission process. This proactive strategy can prevent unnecessary delays and enhance the approval procedure. Additionally, substandard reporting methods, particularly those observed in subgroup analyses, can exacerbate these documentation challenges, underscoring the necessity for thorough reporting in clinical trials.
- Long Review Times: Anticipating potential delays in the evaluation process is essential. Engaging with the regulatory authority early in the INVIMA clinical trial submission process can clarify documentation requirements and expectations, thus helping to avoid misunderstandings that could extend the review period.
- Communication Gaps: Effective communication with the regulatory body and the ethics committee is vital. Establishing and maintaining open lines of communication enables researchers to promptly address any issues. Regular follow-ups can facilitate a smoother submission process, ensuring that all parties are aligned.
- Regulatory Changes: The regulatory landscape is ever-evolving, making it essential for researchers to stay informed about changes that may impact the submission process. Subscribing to updates from the relevant authority and consulting with regulatory experts, such as Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, can aid in navigating these changes effectively. Proactively addressing these challenges can significantly enhance the likelihood of success in the INVIMA clinical trial submission process. For instance, a recent analysis revealed that collaboration between industry and non-industry organizations can increase the probability of success (POS) of drug development projects by 11.3 percentage points. Furthermore, as highlighted by Scott R. Evans, Ph.D., researchers can refer to the Consolidated Standards of Reporting Trials (CONSORT) Statement to alleviate issues stemming from insufficient reporting of randomized controlled studies. By leveraging such partnerships and focusing on detailed documentation and communication, researchers can improve their chances for success in the INVIMA clinical trial submission process. Moreover, the complexities of managing adaptive study designs emphasize the importance of a well-organized submission system capable of addressing these challenges. Bioaccess offers extensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, reporting on serious and non-serious adverse events, and review and feedback on study documents, to assist researchers in effectively navigating these challenges.
Understanding Timelines and Fees for INVIMA Approval
Understanding the timelines and fees associated with the INVIMA clinical trial submission process is crucial for efficient project management in research trials.
-
Approval Timelines: The initial review conducted by INVIMA typically spans 2 to 4 weeks. Following this, the ethics committee review can extend the process by an additional 4 to 6 weeks. Consequently, researchers should anticipate a comprehensive approval timeline of approximately 2 to 3 months. This timeframe is essential for planning and coordinating subsequent stages of the research study, ensuring that all stakeholders are aligned and informed. Significantly, grasping these timelines is increasingly vital as 20% of high-risk therapeutic medical products were initially introduced in the US from 2013 to 2023, underscoring the competitive environment and the necessity for prompt approvals.
-
Fees: INVIMA's fee structure is contingent upon the complexity of the research application. Researchers should expect to allocate between USD $1,000 and $5,000 for these fees, which vary according to the project's scope and specific requirements. Understanding these expenses in advance facilitates improved financial organization and resource distribution, which is essential for the successful implementation of research studies. As highlighted by Kushal T. Kadakia, MSc, having complete access to all information and taking responsibility for its integrity is paramount in research studies, emphasizing the significance of precise budgeting and planning.
-
Comprehensive Research Management Services: At bioaccess, we offer a complete range of services to support research studies in Colombia and across Latin America. Our capabilities encompass feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting. By proactively scheduling these timelines and related expenses, researchers can enhance their project management strategies, ensuring smoother navigation through the INVIMA clinical trial submission process. Furthermore, the regulatory oversight illustrated by the CBER review procedure case study serves as a reminder of the challenges producers face in ensuring compliance, which is pertinent to the approval process. Additionally, the decline in items authorized by the 510(k) pathway indicates a growing reliance on more rigorous approval methods, further underscoring the importance of understanding the INVIMA clinical trial submission process.
Conclusion
Navigating the complexities of clinical trials in Colombia necessitates a thorough understanding of the regulatory landscape shaped by INVIMA. As the primary authority overseeing medical product safety and efficacy, INVIMA is pivotal in the approval process, which encompasses meticulous reviews of clinical trial protocols, adherence to Good Clinical Practice (GCP), and the safeguarding of participant rights. Familiarity with INVIMA's functions and the essential documentation required for submissions is indispensable for researchers striving for successful trial approvals.
Furthermore, grasping key regulations such as Resolution 2378 of 2014 and Decree 1787 of 2014 is crucial for upholding ethical standards and ensuring compliance throughout the trial process. With Colombia emerging as a cost-effective destination for clinical studies—propelled by INVIMA's proactive initiatives—researchers can capitalize on this advantageous environment to advance medical knowledge while adhering to robust regulatory frameworks.
By adhering to a structured approval process and addressing common challenges like incomplete documentation and communication gaps, researchers can significantly enhance their chances of success. Engaging with specialized services, such as those provided by bioaccess, offers invaluable support in navigating these complexities, from feasibility studies to trial management.
Ultimately, remaining informed about INVIMA’s evolving regulations and maintaining compliance throughout the trial lifecycle not only fosters trust with regulatory authorities but also contributes to the integrity and safety of clinical research. As Colombia continues to position itself as an attractive hub for clinical trials, researchers equipped with the right knowledge and resources will be well-prepared to thrive in this competitive landscape.
Frequently Asked Questions
What is the role of the National Food and Drug Surveillance Institute (INVIMA) in Colombia?
INVIMA serves as Colombia's principal regulatory authority responsible for ensuring the safety, effectiveness, and quality of medical products, including those related to research studies. It evaluates research study applications, supervises ongoing investigations, and ensures compliance with national and international regulations.
What are the key responsibilities of INVIMA?
INVIMA's key responsibilities include reviewing clinical trial protocols for ethical and scientific standards, monitoring compliance with Good Clinical Practice (GCP) guidelines, and safeguarding the rights and well-being of research participants.
What is the process for submitting a research study application to INVIMA?
The submission process involves an initial evaluation, a comprehensive expert review, and final approval by a higher-level committee, which ensures high standards in medical research.
What services does bioaccess provide to facilitate the INVIMA clinical trial submission process?
Bioaccess offers extensive management services, including feasibility assessments, site selection, compliance evaluations, study preparation, import permits, nationalization of investigational devices, project oversight, and reporting on study status, inventory, and adverse events.
How does conducting medical studies in Colombia compare to the U.S. in terms of cost?
Medical procedures in Colombia can be 40% to 75% less expensive than in the U.S., allowing for more extensive studies without compromising quality.
What essential documents are required for the INVIMA clinical trial submission process?
Required documents include: 1. Clinical Trial Protocol 2. Informed Consent Form (ICF) 3. Investigator's Brochure 4. Ethics Committee Approval 5. Financial Disclosure 6. Insurance Certificate 7. Application Form.
Why is the accurate completion of submission documents important?
Accurate and comprehensive completion of these documents is crucial for the success of the INVIMA clinical trial submission process, as common errors can lead to delays or rejections.
What is the significance of the Statistical Analysis Plan (SAP) in the submission process?
The SAP is essential for establishing main endpoints before onboarding the first patient and ensuring clarity and focus throughout the study. Transparency in the SAP enhances the integrity of study execution and reporting.
How can researchers improve their chances of a successful application for approval?
By adhering to guidelines, ensuring meticulous preparation of documentation, and transforming experimental designs into actionable strategies, researchers can significantly improve their chances of a successful application.




