Introduction
The 510(k) submission process represents a pivotal step for medical device manufacturers seeking to navigate the regulatory landscape established by the FDA. This pathway allows companies to demonstrate that their devices are substantially equivalent to existing, legally marketed products, ensuring safety and efficacy before reaching the market.
With an increasing focus on global expansion, particularly in markets such as Japan and China, understanding the nuances of the 510(k) process has never been more critical. From identifying appropriate predicate devices to preparing comprehensive documentation, each stage of the submission requires meticulous attention to detail.
As industry leaders emphasize the importance of compliance, this article delves into the complexities of the 510(k) submission process, offering insights and best practices to enhance the likelihood of successful outcomes.
Understanding the 510(k) Submission Process
The 510k FDA clearance application procedure is a vital regulatory route established by the FDA, enabling medical product manufacturers to demonstrate the safety and effectiveness of their offerings by showing substantial equivalence to legally marketed items. This pathway is particularly vital for Class II and III products, as well as select Class I items, which must adhere to design controls as outlined in 21 CFR 820.30. Comprehending the complexities of the 510(k) application is crucial for effectively navigating the 510k FDA clearance procedure, particularly given that 23 percent of industry leaders have noted a strategic emphasis on entering the Japanese and Chinese markets—areas where adherence to 510(k) standards is vital for achieving 510k FDA clearance for market access.
Key elements of the 510(k) process include:
- The categories of products that necessitate 510k FDA clearance, which generally encompass:
- Most Class II products
- Certain Class III products
- Specific Class I items
The documentation needed for a 510k FDA clearance typically includes:
- Comprehensive descriptions of the apparatus
- Labeling details
- Substantial equivalence data
All of which must be carefully prepared to comply with FDA standards.
Timelines for application and review can differ, but a clear understanding of these factors can greatly improve the chances for a favorable outcome. The FDA believes that adherence to best practices outlined in their guidance will promote the development of safer and more effective medical products, facilitating the process of obtaining 510k FDA clearance within the 510(k) Program. Furthermore, the case study of the withdrawal of accreditation from Accelerated Device Approval Services, LLC illustrates the importance of compliance, as the FDA has the authority to suspend or withdraw accreditation from organizations that do not meet regulatory requirements.
Recent updates in 2024 concerning application processes emphasize the significance of remaining aware of changing requirements and expectations, especially considering new guidelines that may influence application strategies.
By thoroughly addressing these factors, professionals engaged in clinical research can enhance their readiness for the intricacies of the 510k FDA clearance application process, with support from experts such as Ana Criado, a leader in Regulatory Affairs with extensive experience in biomedical engineering and health economics, and Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics. Their insights highlight the importance of understanding Regulatory frameworks not only in Colombia but also in international markets.
Step-by-Step Guide to Navigating the 510(k) Clearance Process
- Determine the need for a 510k FDA clearance by assessing whether your product necessitates a 510(k) submission. An instrument is regarded as substantially equivalent if it has the same intended use and technological characteristics as the predicate instruments. This involves a thorough comparison of your equipment with existing products in the market, a process guided by the expertise of regulatory leaders like Ana Criado, who has extensive experience in navigating these requirements, particularly in the context of cannabis-related equipment.
- Identify Predicate Instruments: Conduct in-depth research to select appropriate predicate instruments that closely resemble your product. This identification is essential, as the FDA requires evidence of substantial equivalence to these predicate products for 510k FDA clearance. Regulatory consultants such as Ana Criado, who has worked with global companies, can provide invaluable insights in this phase, drawing from her experience in ensuring compliance for cannabis-based products.
- Prepare Required Documentation: Assemble all necessary documentation, which should include a detailed description of the equipment, labeling information, performance data, and a comprehensive risk analysis. This documentation forms the backbone of your entry, demonstrating the device's safety and efficacy. Ana’s experience as a professor in biomedical engineering can aid in ensuring that your documentation meets academic and regulatory standards. As Ana frequently mentions, "Thorough documentation not only aids in presentation but also enhances product credibility in the market."
- Draft the 510(k) FDA clearance Application: Organize your compiled documentation into a coherent format that meets FDA requirements. Clarity and precision are paramount; any deviations from published guidance must be substantiated with scientifically justified alternatives to avoid requests for Additional Information (AI) during the review process. Experts like Ana Criado underscore the importance of being prepared to substantiate any deviations to avoid delays.
- Submit the 510k FDA clearance: Utilize the FDA’s electronic filing system to submit your completed 510(k) for the necessary clearance. Ensure that all components are thoroughly checked for completeness and compliance before submission. It is important to note that a recent survey revealed that nearly 90 percent of medical device industry leaders prioritize US regulatory approval, underscoring the significance of this procedure.
- Respond to FDA Queries: Be ready to address any inquiries or requests for additional information from the FDA throughout their review. Timely and thorough responses are critical to maintaining momentum toward clearance. Engaging a regulatory consultant can help streamline this communication. Ana emphasizes that "Effective communication with the FDA is crucial for a smooth review."
- Upon receiving 510k FDA clearance, transition your focus to compliance with any post-market surveillance requirements. This marks a significant milestone, allowing you to market your product in the U.S. while ensuring adherence to safety regulations.
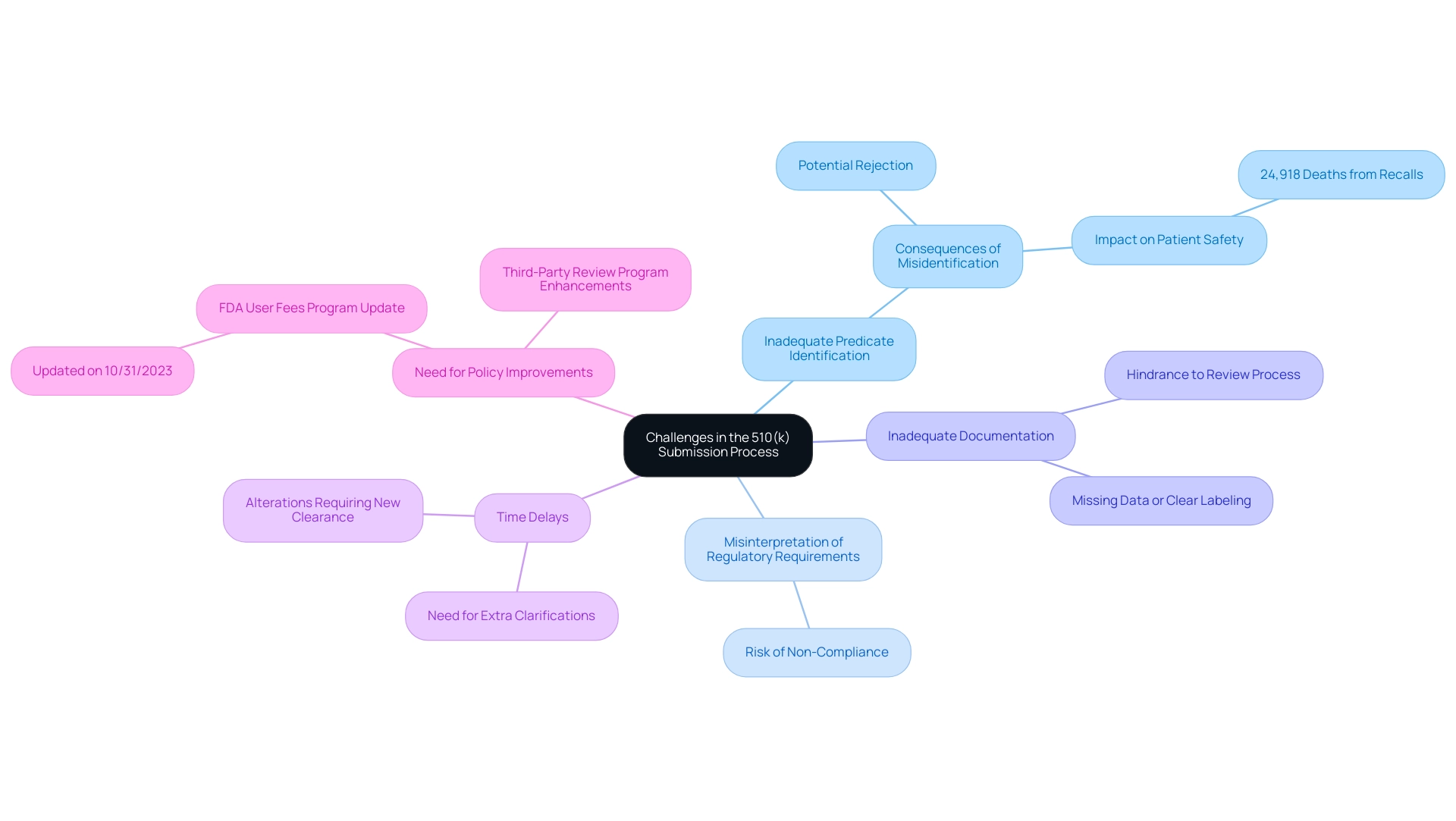
Common Challenges in the 510(k) Submission Process
Navigating the 510k FDA clearance application process presents several major challenges that can hinder the approval of medical products. Among these, the inadequate identification of predicate items stands out as a critical issue. Failing to accurately select a suitable predicate can lead to outright rejection, as obtaining a 510k FDA clearance requires clear evidence of substantial equivalence.
This challenge is compounded by the potential for misinterpretation of regulatory requirements, which can result in non-compliance with FDA guidelines. Moreover, inadequate documentation often affects entries; those missing thorough data or clear labeling are commonly sent back for more information, thus hindering the review process.
Time delays can also arise from the need for extra clarifications, which extends the overall timeline for approval. Moreover, any alterations made to equipment designs during the application may require a new 510k FDA clearance or necessitate additional documentation, further complicating the situation. The FDA's Reduced Medical Equipment User Fees program for small businesses, updated on 10/31/2023, aims to ease some financial pressures, yet many manufacturers still encounter difficulties in navigating the filing system efficiently.
As highlighted in recent inquiries documented in JAMA, which examined the repercussions of inadequate predicate equipment identification, there were 24,918 deaths associated with recalls from the MAUDE database between 2003 and 2020. This statistic underscores the critical importance of precise identification in ensuring patient safety and regulatory compliance. Industry leaders, including Brian J. Miller, emphasize the need for policy improvements in the third-party review program to enhance the efficiency of FDA medical product regulation, noting,
Finally, the article reviews potential policy improvements to the third party review program designed to improve the efficiency of FDA medical product regulation.
To further illustrate the challenges faced, a case study titled 'Optimizing Medical Product Pricing and Reimbursement Strategies' demonstrates how utilizing market intelligence can enhance financial performance and market access for medical products. By recognizing these typical traps in the 510(k) application procedure, manufacturers can enhance their proposals, ultimately boosting their likelihood of obtaining 510k FDA clearance.
Demonstrating Substantial Equivalence: Key to Successful 510(k) Submissions
To demonstrate substantial equivalence for 510k FDA clearance, manufacturers must present compelling evidence that their product matches the safety and effectiveness of a predicate. This rigorous process involves several key steps:
-
Comparative Analysis: Conduct an in-depth comparative analysis that examines your device's intended use, technological characteristics, and performance metrics alongside those of the predicate device.
Effective comparative analysis is essential for establishing the foundation of equivalence. For instance, the recent approval of Samsung Bioepis and Biogen’s Aflibercept biosimilar by the European Commission highlights the significance of demonstrating equivalence in a competitive market.
-
Data Collection: Assemble robust data supporting your equivalence claims, which should include clinical data, results from bench testing, and any relevant empirical evidence.
This data is crucial in validating your assertions and enhancing the credibility of the submission.
-
Documentation: Thoroughly document your findings within the 510(k) application. Ensure that all comparisons are articulated clearly and substantiated with appropriate evidence.
Clarity in documentation can facilitate the review process and assist reviewers in understanding the rationale behind your equivalence claims.
-
Regulatory Guidance: Adhere to FDA guidance documents that outline the requirements for demonstrating substantial equivalence. Familiarity with these guidelines is vital for ensuring compliance and can significantly influence the success of the proposal.
Additionally, it is important to note that to claim a preamendments device, a firm must demonstrate that the device was marketed for a specific intended use that has not changed.
This approach is integral to obtaining 510k FDA clearance and can substantially affect the review outcome, underscoring the necessity for meticulous preparation and attention to detail. Utilizing the comprehensive clinical trial management services provided by bioaccess®, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, along with services such as trial setup, project management, and regulatory compliance reviews conducted by specialists like Katherine Ruiz, can greatly enhance this workflow. As Jon Speer, Founder & VP QA/RA at Greenlight Guru, highlights, 'The clarity and rigor of your application can make all the difference in the FDA's evaluation.
Best Practices for a Successful 510(k) Submission
- Early Engagement with the FDA: Initiating pre-submission meetings with the FDA can provide invaluable insights into the expectations for your submission. Such engagement allows for constructive feedback on your strategy, increasing the likelihood of approval. Trey Thorsen, a regulatory consultant, highlights the importance of this phase by stating, "Need expert help navigating the premarket notification process?" This underscores the value of professional guidance during this critical stage, particularly with experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia. Katherine's extensive experience in clinical trial management, including feasibility studies and compliance reviews, is crucial for ensuring a successful application.
- Thorough Documentation: Comprehensive and organized documentation is vital for a successful presentation. The inclusion of Declarations of Conformity and Summary Reports in Section 9 demonstrates adherence to specific standards, thus avoiding an incomplete presentation appearance that could delay review. Furthermore, a case study titled 'Impact of Prevalence on Agreement Measures' illustrates how the prevalence of a condition can significantly influence agreement measures between a new test and a non-reference standard. This highlights the significance of taking prevalence into account when analyzing documentation and filing methods.
- Clear Communication: Establishing open lines of communication with the FDA and promptly addressing any inquiries fosters a cooperative relationship, aiding in the review of materials.
- Quality Control: A robust quality control system is essential. Before sending, all materials should undergo meticulous review to minimize errors, ensuring that the integrity of the entry is upheld. Experts like Ana Criado, with her extensive background in Regulatory Affairs and biomedical engineering, are invaluable in maintaining these quality standards. Furthermore, Ana's responsibility in supervising compliance evaluations and trial arrangements guarantees that all entries satisfy the necessary regulatory standards.
- Continuous Learning: Staying abreast of evolving FDA regulations and guidelines is crucial for compliance. This proactive approach allows you to adapt to new requirements effectively. Recent statistics show that the overall percent agreement calculated from revised results was a notable 99.5% (219/220), underscoring the impact of thorough documentation on the evaluation process. By adhering to these best practices and recognizing that the 510(k) submission serves as a guide for medical device makers to ensure safety and effectiveness, you can enhance the probability of a successful 510(k) submission, thereby facilitating faster market access for your medical device.
Conclusion
Navigating the 510(k) submission process is essential for medical device manufacturers aiming to secure FDA clearance and successfully enter the market. Understanding the intricacies of this regulatory pathway, from identifying appropriate predicate devices to preparing comprehensive documentation, is critical. As highlighted, the importance of demonstrating substantial equivalence cannot be overstated, as it serves as the foundation for a successful submission.
Moreover, the challenges faced during this process, including inadequate identification of predicate devices and insufficient documentation, underscore the necessity for meticulous preparation. Engaging with regulatory experts and adhering to best practices such as:
- Early FDA communication
- Thorough documentation
- Quality control
can significantly enhance the likelihood of a favorable outcome.
Ultimately, the 510(k) submission process is not just a regulatory hurdle but a vital step toward ensuring the safety and efficacy of medical devices. By prioritizing compliance and staying informed about evolving guidelines, manufacturers can facilitate faster market access for their innovations, ultimately benefiting patients and the healthcare community at large.
Frequently Asked Questions
What is the 510(k) FDA clearance application procedure?
The 510(k) FDA clearance application procedure is a regulatory pathway established by the FDA that allows medical product manufacturers to demonstrate the safety and effectiveness of their products by showing substantial equivalence to legally marketed items.
Which product classes typically require 510(k) FDA clearance?
The 510(k) FDA clearance is generally required for most Class II products, certain Class III products, and specific Class I items.
What documentation is needed for a 510(k) FDA clearance application?
Required documentation includes comprehensive descriptions of the device, labeling details, and substantial equivalence data, all of which must comply with FDA standards.
How do timelines for the 510(k) application and review process vary?
Timelines can differ based on several factors, but understanding these factors can significantly improve the chances of a favorable outcome.
What recent updates in 2024 should applicants be aware of?
Recent updates emphasize the importance of staying informed about changing requirements and expectations, as new guidelines may influence application strategies.
What are the key steps in the 510(k) FDA clearance application process?
Key steps include determining the need for clearance, identifying predicate instruments, preparing required documentation, drafting the application, submitting the application, responding to FDA queries, and ensuring compliance with post-market surveillance requirements.
Who can assist in navigating the 510(k) application process?
Experts such as Ana Criado, a leader in Regulatory Affairs, and Katherine Ruiz, a specialist in Regulatory Affairs for medical products, can provide valuable insights and support throughout the process.
What is the significance of compliance in the 510(k) process?
Compliance is crucial, as the FDA has the authority to suspend or withdraw accreditation from organizations that do not meet regulatory requirements, highlighting the importance of adhering to best practices.




