Overview
The article focuses on mastering the clinical trial approval process in Chile by thoroughly understanding the regulatory landscape and requirements. It emphasizes that successful navigation of this process necessitates comprehensive preparation of documentation and proactive communication with regulatory bodies.
- The Instituto de Salud Pública (ISP)
- Ethics committees
play crucial roles in ensuring compliance with national health standards and ethical considerations. By recognizing these integral components, stakeholders can effectively maneuver through the complexities of clinical research in Chile.
Introduction
Chile is rapidly establishing itself as a key player in the global clinical trial landscape, underscored by a significant uptick in research activities and a robust regulatory framework. In 2025 alone, over 150 clinical trials were conducted, highlighting the country's well-developed healthcare infrastructure and ethical oversight that are paving the way for innovative medical research.
However, navigating this complex environment necessitates a deep understanding of local regulations, the required documentation, and strategic collaboration with local institutions. As the demand for clinical trials continues to rise, stakeholders must remain informed about the evolving landscape to ensure successful outcomes that align with both regulatory requirements and local health priorities.
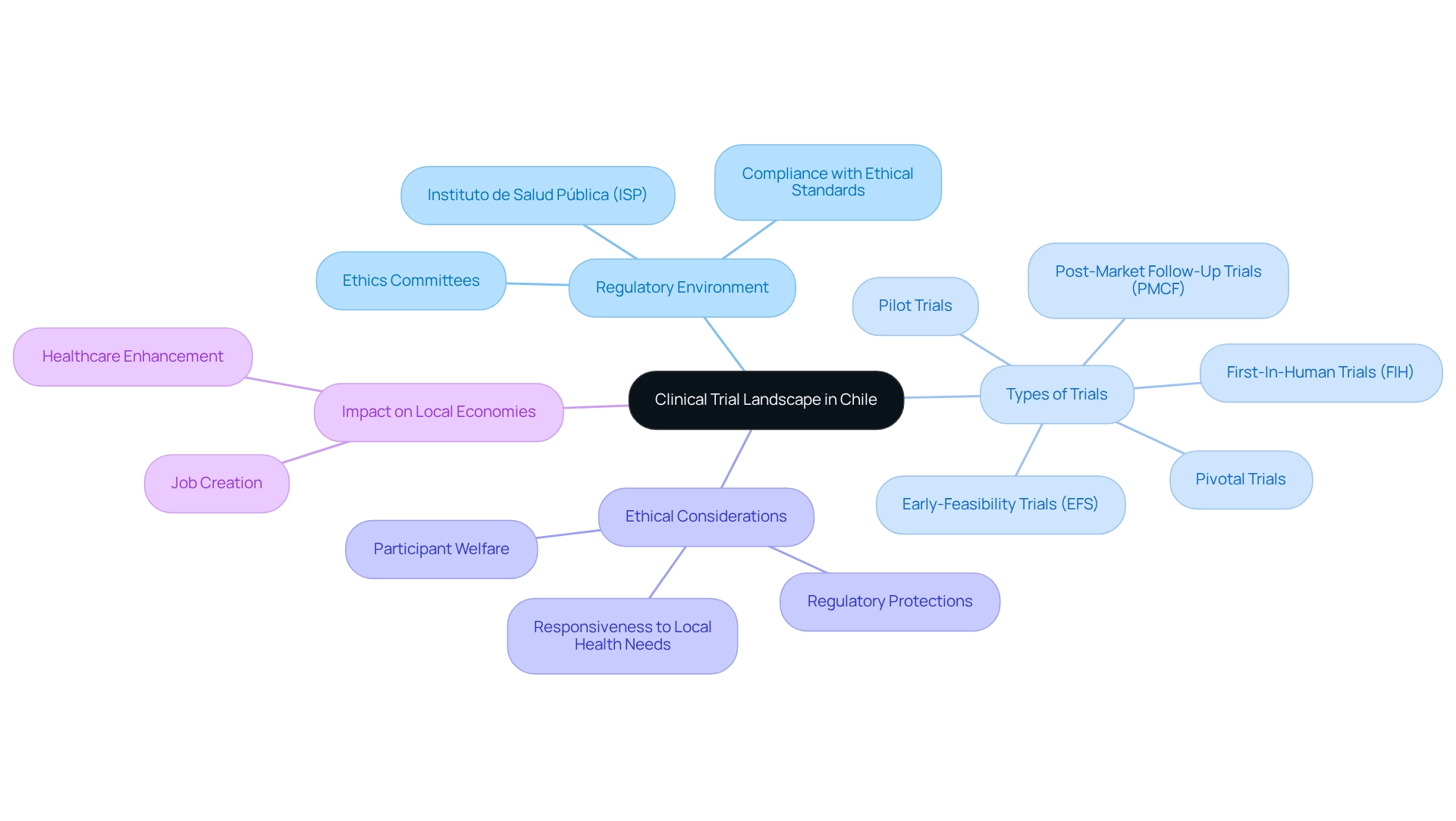
Understand the Clinical Trial Landscape in Chile
Chile has emerged as a notable location for research studies, driven by its well-established healthcare system and supportive regulatory framework. In 2025, the nation observed a significant rise in medical studies, with over 150 research experiments carried out, particularly in Phase I, II, and III stages, indicating a strong growth trend in research endeavors. The regional healthcare system, comprising a network of hospitals and research facilities, plays an essential role in supporting these studies.
The regulatory environment in Chile is primarily overseen by the Instituto de Salud Pública (ISP) along with several ethics committees that supervise the clinical trial approval process and monitoring procedures. This regulatory oversight ensures that research complies with ethical standards while being attentive to local health requirements. Understanding the categories of assessments carried out—from early-feasibility evaluations to pivotal experiments—is crucial for aligning research objectives with the capabilities and expectations of the Chilean healthcare system.
bioaccess® brings over 20 years of expertise in Medtech, focusing on managing a range of trials, including:
- Early-Feasibility Trials (EFS)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Follow-Up Trials (PMCF)
This knowledge is essential for navigating the complexities of the clinical trial approval process in Chile, ensuring that each study is tailored to meet both regulatory standards and local health priorities.
Recent evaluations highlight the significance of ethical considerations in research studies, especially in light of evolving regulatory safeguards. As Bernardo Aguilera notes, "This investigation can shed light on how international research is being conducted in Chile, the effects of new regulatory protections, and how responsive the research is to local health needs and priorities." These frameworks prioritize participant welfare and ensure that studies align with the health priorities of the local population. Furthermore, efficient oversight strategies examined in Argentina can provide valuable perspectives for enhancing research outcomes in Chile. As the research landscape in Chile continues to evolve, staying attuned to contemporary trends and successful study examples will be crucial for navigating this dynamic environment. Moreover, the impact of these medical investigations on local economies, encompassing job creation and healthcare enhancement, underscores the broader significance of research in the region.
Identify Regulatory Requirements and Key Agencies
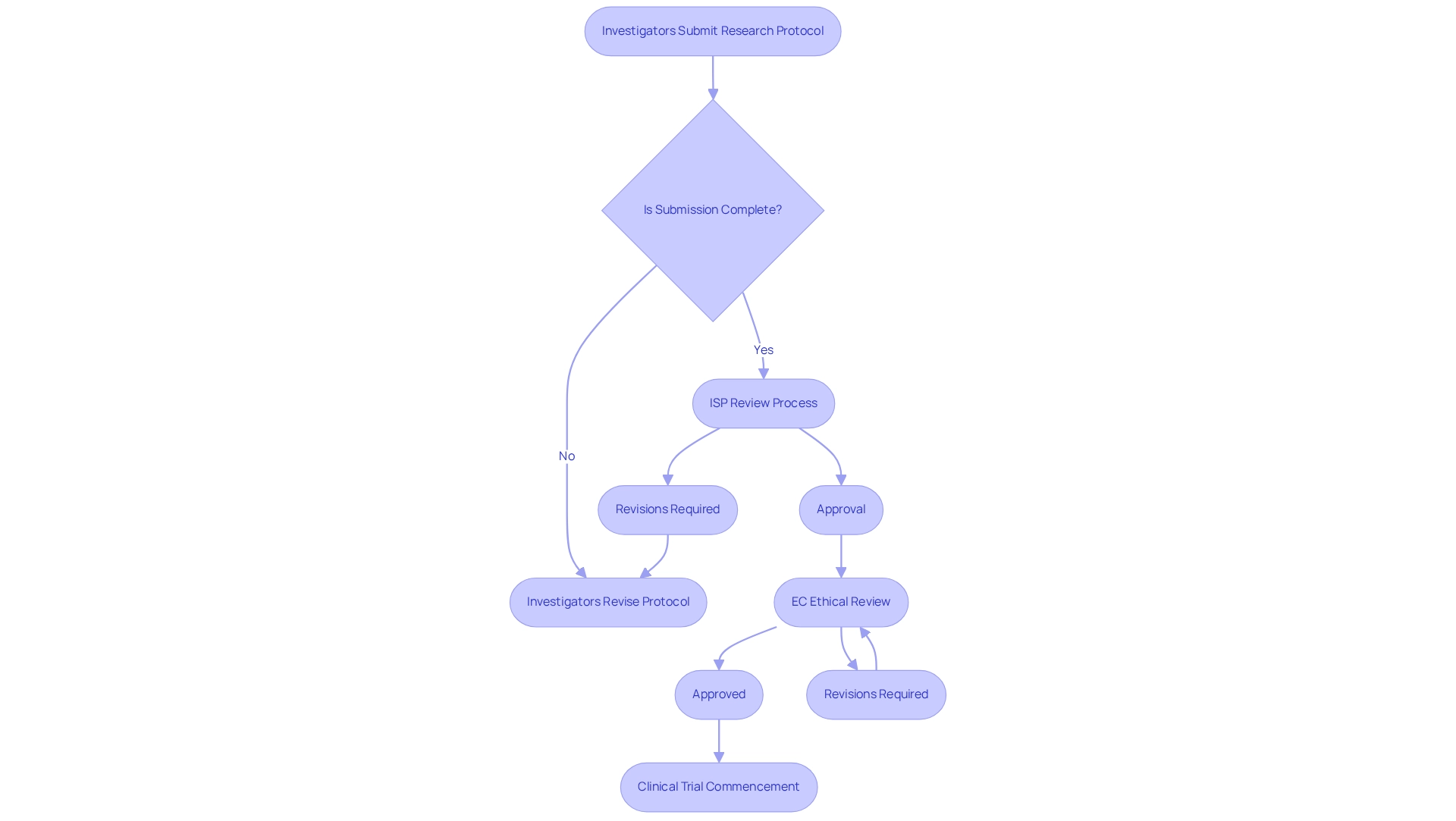
In Chile, the Instituto de Salud Pública (ISP) serves as the primary regulatory authority overseeing the clinical trial approval process, ensuring that all investigations align with national health standards. A pivotal component of this process is the Ethical-Scientific Evaluation Committee (EC), which is tasked with the thorough review and endorsement of study protocols.
To initiate a medical research study, investigators are required to submit a comprehensive research protocol that outlines the objectives, methodologies, and ethical considerations involved. This submission must also include a robust informed consent procedure for participants, prioritizing their rights and data protection in accordance with local regulations.
At bioaccess®, we are committed to safeguarding information security and fostering client trust throughout the clinical study. Our grievance and data protection protocols are meticulously designed to address client concerns with compliance and transparency, reinforcing our dedication to ethical practices.
Understanding the specific requirements set forth by the ISP and the EC is essential for navigating the clinical trial approval process in Chile, as these can differ based on the study type and the medical products being investigated. Notably, the regulatory landscape is evolving, with updates expected in 2025 that may influence the clinical trial approval process in Chile.
Between 2010 and 2019, a total of 876 studies were registered in Chile, highlighting the dynamic nature of the research environment. Collaborating with local universities and research institutions can significantly enhance research feasibility and resource availability, ultimately leading to improved health outcomes.
Our extensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
Each designed to facilitate successful experiments. Furthermore, recent research indicates that the increasing trend of outsourcing medical tests is driven not only by financial considerations but also by challenges in patient enrollment within domestic regions. As noted by Maryam Kermanimojarad, this research provides policymakers with new insights into the developmental challenges facing the pharmaceutical sector, underscoring the importance of strategic planning in research initiatives.
Prepare and Submit Required Documentation
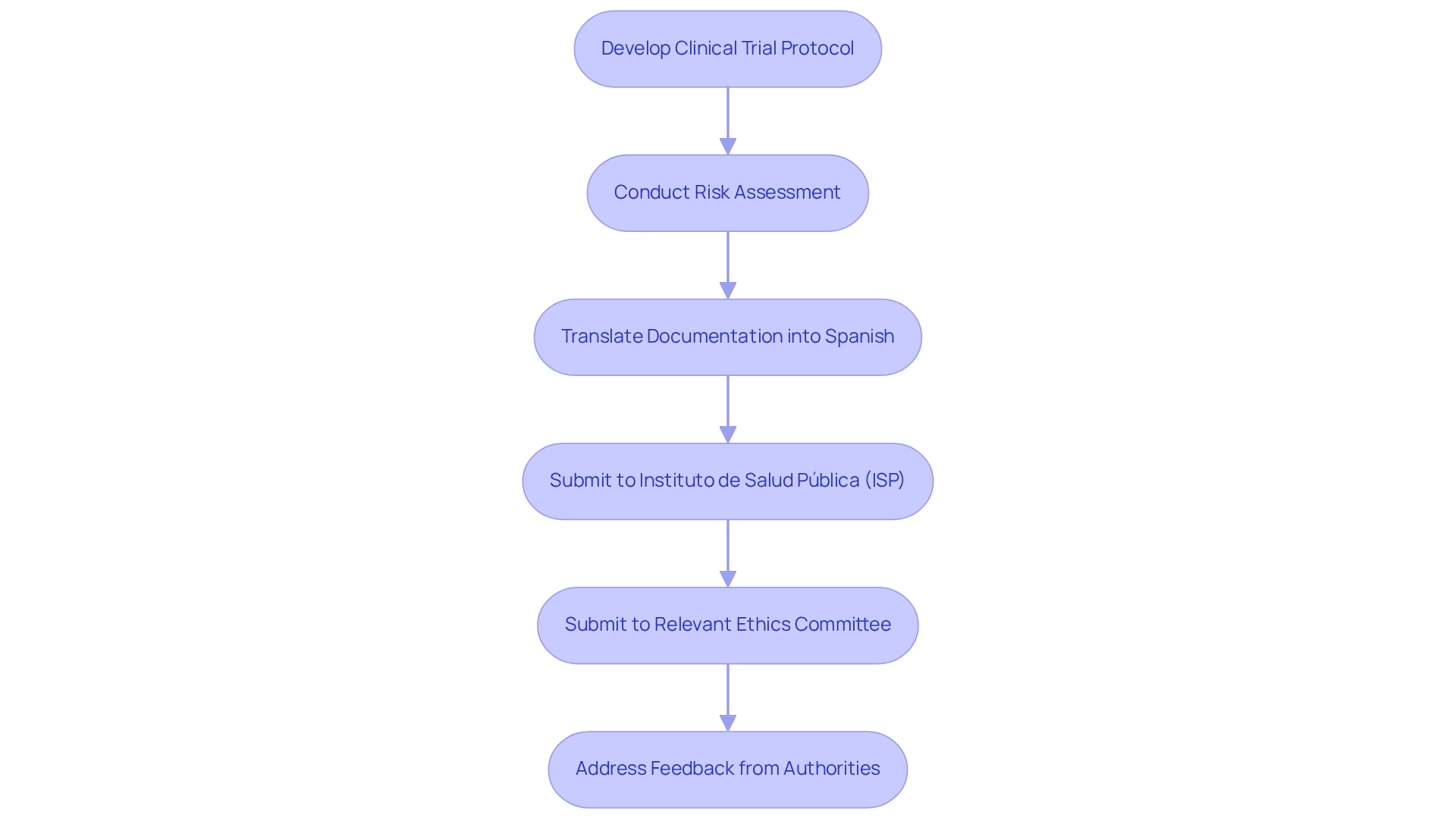
To ensure a successful submission in the clinical trial approval process in Chile, it is essential to develop a comprehensive clinical trial protocol that clearly defines the study's objectives, design, methodology, and statistical analysis plan. A thorough risk assessment must be included, along with proof of ethical approval from an accredited ethics committee. All documentation should be meticulously translated into Spanish, as this is a mandatory requirement for submission. Once the documentation is finalized, it should be submitted to the Instituto de Salud Pública (ISP) and the relevant ethics committee. It is essential to stay attentive to any feedback or inquiries for further information from these entities to avoid delays in the approval timeline.
Significantly, GlySure realized savings of 9 to 12 months in development time because of quicker regulatory approval procedures, highlighting the significance of comprehensive preparation. Furthermore, Chile encounters difficulties with outdated research regulations and a complicated clinical trial approval process, which may discourage MedTech firms from conducting studies in the nation. As Maryam Kermanimojarad noted, this study provides policy-decision makers with new insights into the development issues surrounding the pharmaceutical industry. By leveraging the expertise of bioaccess®, which specializes in managing clinical trials across Latin America, companies can navigate these challenges more effectively. Following these best practices not only simplifies the submission procedure but also increases the chances of a successful outcome.
Navigate the Review Process and Address Challenges
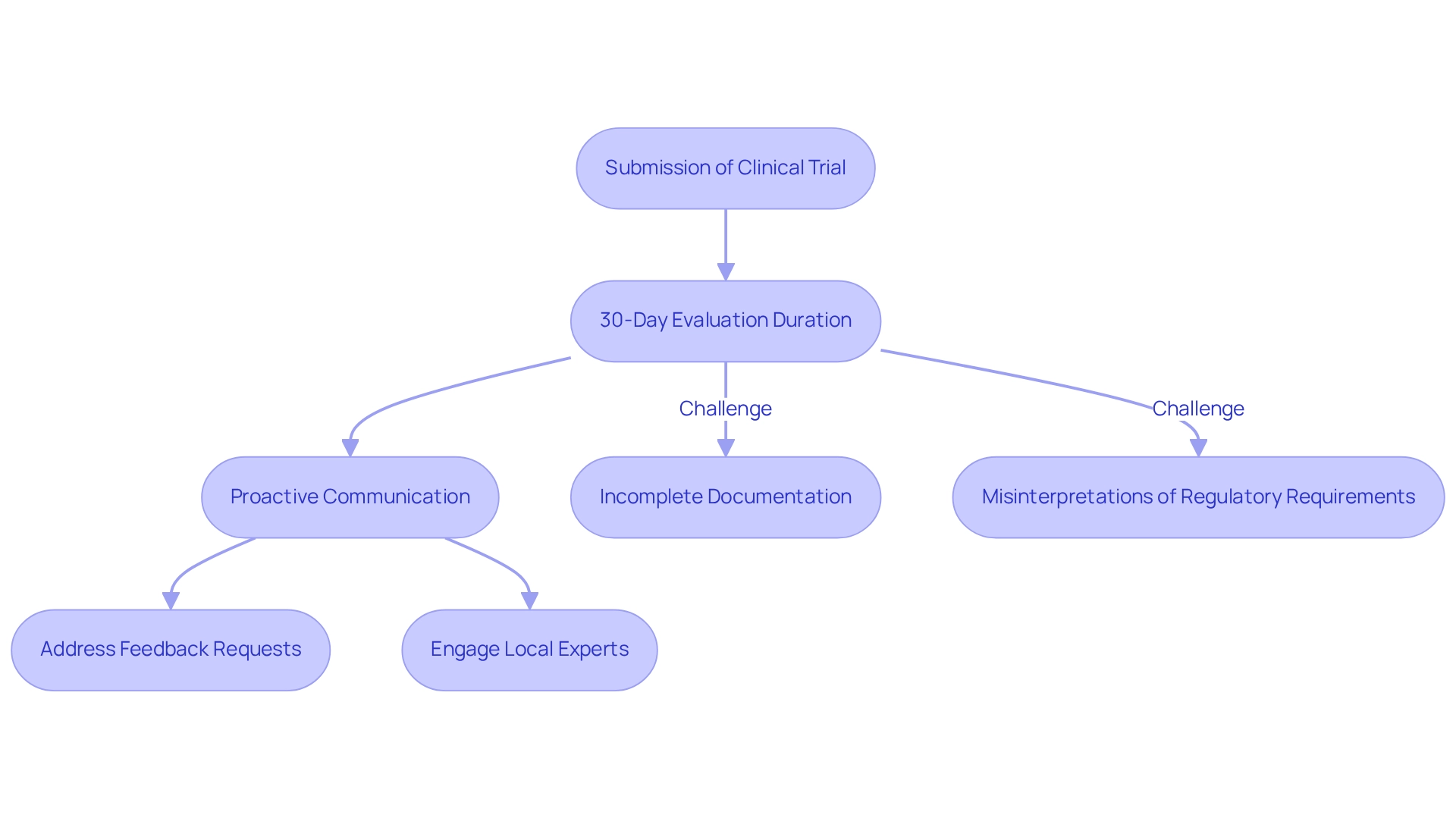
Upon submission, the evaluation duration in the clinical trial approval process in Chile typically lasts about 30 days. During this period, proactive communication with regulatory agencies and ethics committees is crucial. Promptly addressing any feedback or requests for clarification can significantly streamline the process. Common challenges encountered include delays stemming from incomplete documentation or misinterpretations of regulatory requirements. To effectively navigate these hurdles, it is advisable to maintain open lines of communication with the involved agencies and seek guidance from local experts or consultants well-versed in the Chilean regulatory landscape. This proactive approach not only reduces potential problems but also increases the chances of a seamless advancement through the approval process.
Chile has emerged as a leader in research studies, boasting retention rates exceeding 85%, which surpasses global averages. This trend demonstrates an increasing interest in medical device studies within the region, highlighting the significance of grasping the local regulatory landscape. For instance, Candel Medical Company effectively managed the review of studies to create groundbreaking neurorehabilitation devices, showcasing the possibilities for progress in medical technology emerging from Chile. Their experience emphasizes the importance of comprehensive preparation and regional knowledge in addressing typical obstacles encountered during research approvals.
Furthermore, bioaccess® provides extensive management services for studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This ensures that clients are well-prepared to handle the intricacies of the approval pathway. Establishing a three-step ethical framework, as noted by Annette Rid, is essential for addressing significant ethical concerns in research studies. Chile's commitment to healthcare excellence, supported by the expertise of bioaccess®, sets a benchmark for other nations in Latin America and beyond, further emphasizing the importance of navigating the clinical trial approval process effectively.
Conclusion
Chile is rapidly establishing itself as a significant hub for clinical trials, bolstered by its robust healthcare infrastructure and well-defined regulatory framework. The impressive increase in the number of trials conducted, particularly in 2025, underscores the country's potential to advance medical research while addressing local health needs. A thorough understanding of the regulatory landscape, including the roles of the Instituto de Salud Pública and ethics committees, is essential for stakeholders aiming to navigate this environment successfully.
Preparation and submission of comprehensive documentation are critical steps in the clinical trial process. By adhering to local requirements and ensuring meticulous translations, researchers can mitigate delays and enhance the likelihood of timely approvals. Proactive engagement with regulatory bodies throughout the review process can further streamline operations and address common challenges that may arise.
Ultimately, Chile's commitment to ethical research and participant welfare, coupled with the expertise provided by organizations like bioaccess®, positions the nation as a leader in the clinical trial arena. As the landscape continues to evolve, staying informed about regulations, successful trial examples, and local health priorities will be vital for all stakeholders. Embracing these insights not only fosters successful clinical outcomes but also contributes to the broader advancement of healthcare in the region, highlighting Chile's pivotal role in the global medical research community.
Frequently Asked Questions
Why is Chile considered a notable location for research studies?
Chile is recognized for its well-established healthcare system and supportive regulatory framework, which facilitate the conduct of medical research.
How many research studies were conducted in Chile in 2025?
In 2025, Chile observed over 150 research experiments, particularly in Phase I, II, and III stages, indicating significant growth in research activities.
What role does the healthcare system play in supporting research studies in Chile?
The regional healthcare system, which includes a network of hospitals and research facilities, is essential for supporting the various research studies conducted in the country.
Who oversees the regulatory environment for clinical trials in Chile?
The regulatory environment is primarily overseen by the Instituto de Salud Pública (ISP) and several ethics committees that supervise the clinical trial approval process and monitoring.
What types of trials does bioaccess® manage?
bioaccess® manages a range of trials including Early-Feasibility Trials (EFS), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Follow-Up Trials (PMCF).
Why are ethical considerations significant in research studies in Chile?
Ethical considerations are crucial to ensure participant welfare and to align research with local health needs and priorities, especially in light of evolving regulatory safeguards.
How do recent evaluations contribute to research in Chile?
Recent evaluations highlight the effects of new regulatory protections and how responsive research is to local health needs, which can enhance the effectiveness of studies conducted in Chile.
What impact do medical investigations have on local economies in Chile?
Medical investigations contribute to local economies by creating jobs and enhancing healthcare, underscoring the broader significance of research in the region.
What should researchers consider when navigating the clinical trial approval process in Chile?
Researchers should understand the categories of assessments, align their research objectives with the capabilities of the Chilean healthcare system, and stay attuned to contemporary trends and successful study examples.




