Overview
This article examines the advantages of mastering clinical trial regulations in Latin America, with a particular focus on the benefits for conducting medical studies within this region. It highlights that Latin America presents substantial cost savings, expedited approval processes, and a diverse patient population, rendering it an appealing destination for clinical trials. Notably, Colombia's efficient regulatory framework serves as a prime example, complemented by ongoing reforms aimed at bolstering research capabilities.
Introduction
The regulatory landscape for clinical trials in Latin America presents a complex yet promising environment for researchers and sponsors alike. Each country establishes its own regulations, influenced by local health authorities, making it essential to navigate this diverse terrain for successful trial execution.
Colombia, in particular, stands out as an attractive destination, offering significant cost savings, rapid approval processes, and a robust healthcare system that supports patient recruitment.
As the region undergoes transformative reforms aimed at streamlining regulations and improving infrastructure, the potential for innovative clinical research continues to grow.
However, challenges such as regulatory complexities, cultural sensitivities, and economic fluctuations must be carefully managed to unlock the full benefits of conducting clinical trials in Latin America.
Understanding these dynamics is crucial for stakeholders looking to capitalize on the region's unique advantages while ensuring compliance and ethical integrity in their research endeavors.
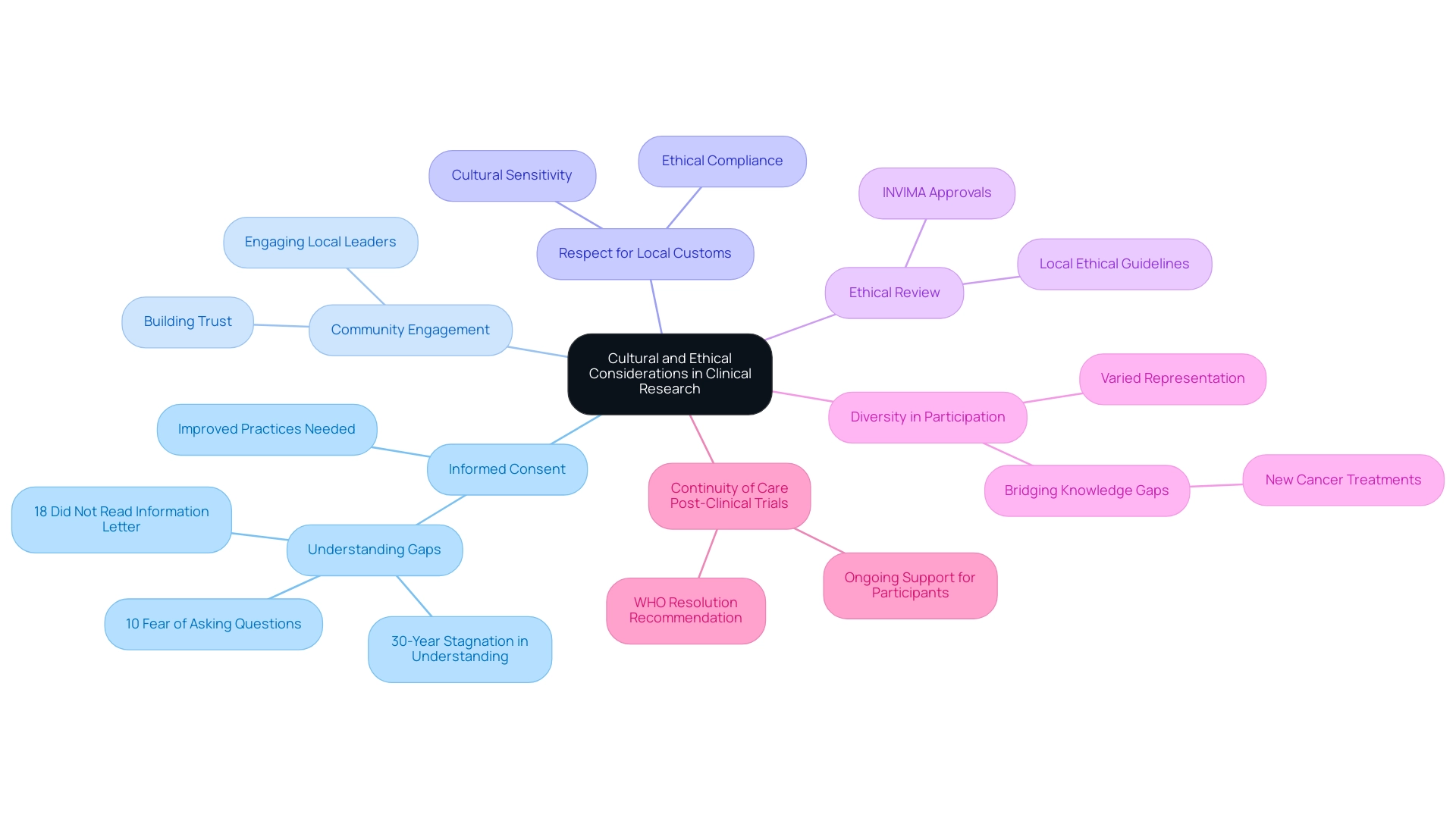
Navigating the Regulatory Landscape for Clinical Trials in Latin America
The regulatory environment for medical studies in Latin America is notably diverse, showcasing significant clinical trial regulation benefits across the region, with considerable differences among various nations. Each country establishes its own distinct regulations, influenced by local health authorities and ethical review boards. In Colombia, the regulatory framework offers several competitive advantages for first-in-human studies, including substantial cost reductions exceeding 30% compared to North America and Western Europe, alongside a swift approval process where IRB/EC and INVIMA assessments are completed within just 90-120 days.
Researchers must become thoroughly acquainted with the guidelines established by INVIMA and the requisite processes, such as obtaining IRB/EC approval, INVIMA approval, and the MinCIT import permit. Familiarity with these regulations is crucial for streamlining approval processes and ensuring compliance with international standards. The high quality of Colombia's healthcare system, ranked among the top five globally by International Living, coupled with its considerable patient recruitment potential—over 50 million individuals, with 95% covered by universal healthcare—positions it as an attractive destination for clinical trials.
Recent reforms in the region are enhancing this landscape, with the aim of simplifying the clinical trial regulation benefits in Latin America. For instance, Brazil's legislative changes allow local ethics committees to independently authorize research protocols, significantly expediting the approval process for multi-site studies. Furthermore, Colombia's ambition to transition into a knowledge-driven economy by 2031 underscores the importance of expanding medical studies in the region.
The evolving regulatory landscape in Colombia, bolstered by R&D tax incentives including generous tax deductions and credits, is facilitating growth through streamlined approval processes and harmonized guidelines, further showcasing the clinical trial regulation benefits in Latin America. Additionally, the varied disease trends, particularly the notable increase in cancer and heart disease, highlight the unique patient demographics that render Latin America a suitable site for trials. This evolution in regulatory practices emphasizes the necessity for investigators to remain vigilant and informed about changes that could impact study timelines and requirements, as the region continues to develop as a center for clinical trials.
Notably, hospitals in Colombia are permitted to conduct research with pharmaceutical drugs only after successfully passing a rigorous ICH/GCP certification process, ensuring high standards of quality and safety. As a reliable CRO, bioaccess® is well-equipped to support researchers in navigating these challenges and accelerating their studies in Colombia.
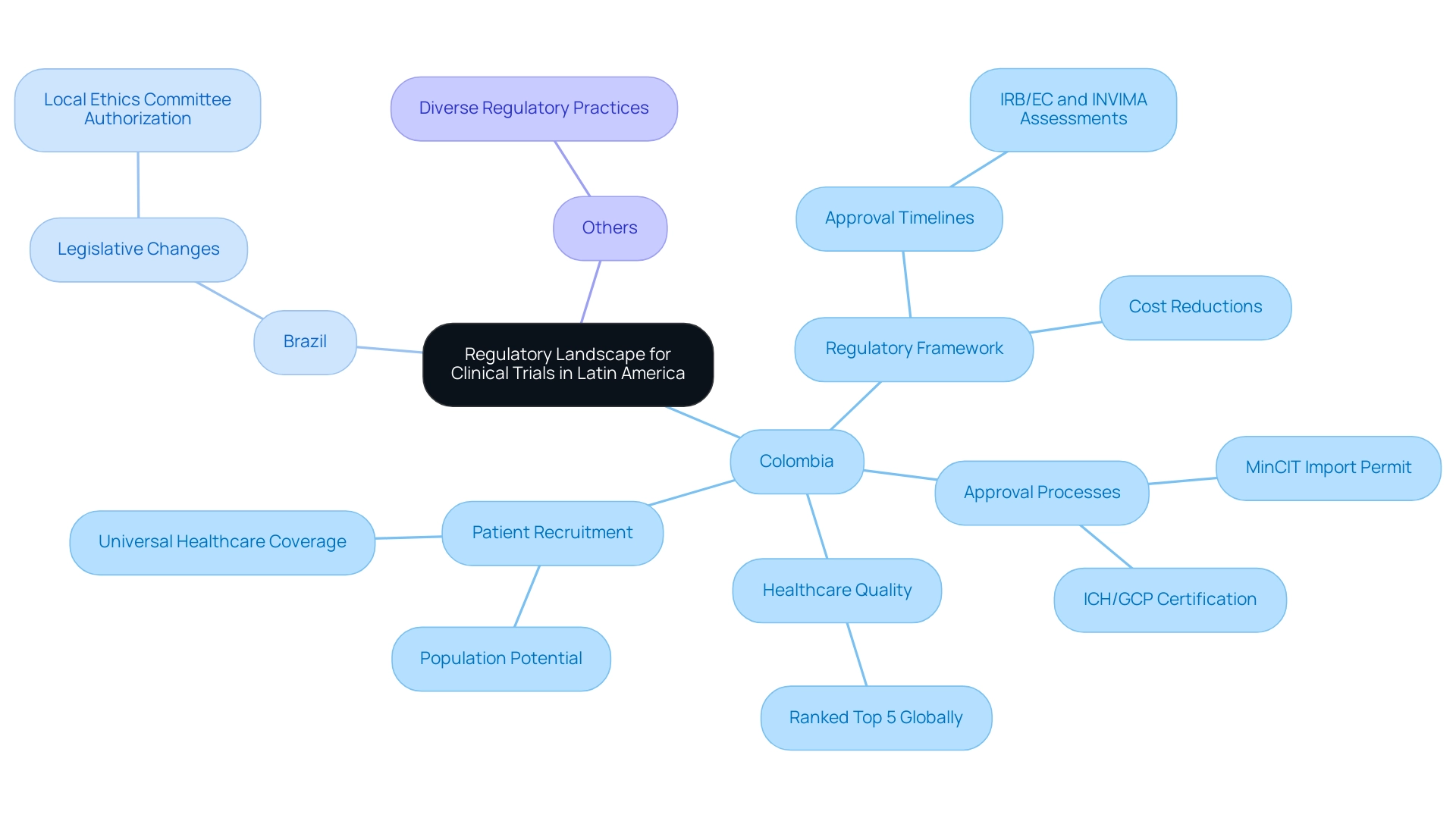
Key Advantages of Conducting Clinical Trials in Latin America
Latin regions present a multitude of strategic advantages for conducting research studies, particularly due to the clinical trial regulation benefits in Latin America, which render it a compelling choice for both investigators and sponsors. Key benefits include:
- Cost Efficiency: Clinical trials in Latin America can yield substantial financial savings compared to North America or Europe, with estimates suggesting potential reductions exceeding 30% in overall costs. This cost-effectiveness is pivotal, especially considering the average development costs for AM drugs, which hover around $144.5 million. Notably, the U.S. government grant funding received by Zemdri is estimated at $220 million, underscoring the significant financial investments inherent in research.
- Diverse Patient Populations: The region is distinguished by its rich ethnic diversity, which enhances the representation of various demographics in research studies. As Gelman articulates, "In virtually any statistical application, it is natural to object to exchangeability on the grounds that the units actually differ." Such diversity is essential for accurately assessing drug efficacy and safety across different populations.
- Faster Recruitment: The robust doctor-patient relationships prevalent in many Latin American countries facilitate higher recruitment rates and enhance participant compliance, ensuring that studies progress swiftly. Insights from the case study titled "Impact of Patient Enrollment on Development Costs" reveal that high enrollment numbers can significantly influence development costs, making recruitment strategies a key focus for financial feasibility. Notably, collaborations like that of bioaccess™ with GlobalCare Clinical Trials have achieved over a 50% reduction in recruitment time and 95% retention rates, showcasing effective strategies in action.
- Enhancing Infrastructure: Continuous investments in healthcare and research facilities throughout the region are strengthening the capacity to conduct high-quality medical studies, which is vital for meeting international standards. The collaboration between bioaccess™ and Caribbean Health Group aspires to establish Barranquilla as a prominent location for medical studies in Latin regions, supported by efforts from Colombia's Minister of Health, who has publicly endorsed this partnership during a gathering in Miami, FL.
- Regulatory Reforms: Recent reforms in regulatory frameworks have led to clinical trial regulation benefits in Latin America by streamlining approval processes, significantly reducing bureaucratic hurdles and expediting timelines for study initiation. This evolving landscape positions the Latin region as a promising center for groundbreaking medical studies, as illustrated by insights shared by industry leaders such as Dushyanth Surakanti, the Founder & CEO of Sparta Biomedical, during their initial human assessment with bioaccess®. Furthermore, the partnership with IDx Technologies aims to identify Latin American ophthalmology centers for AI-driven disease detection, thereby enhancing the region's capabilities in scientific investigation.
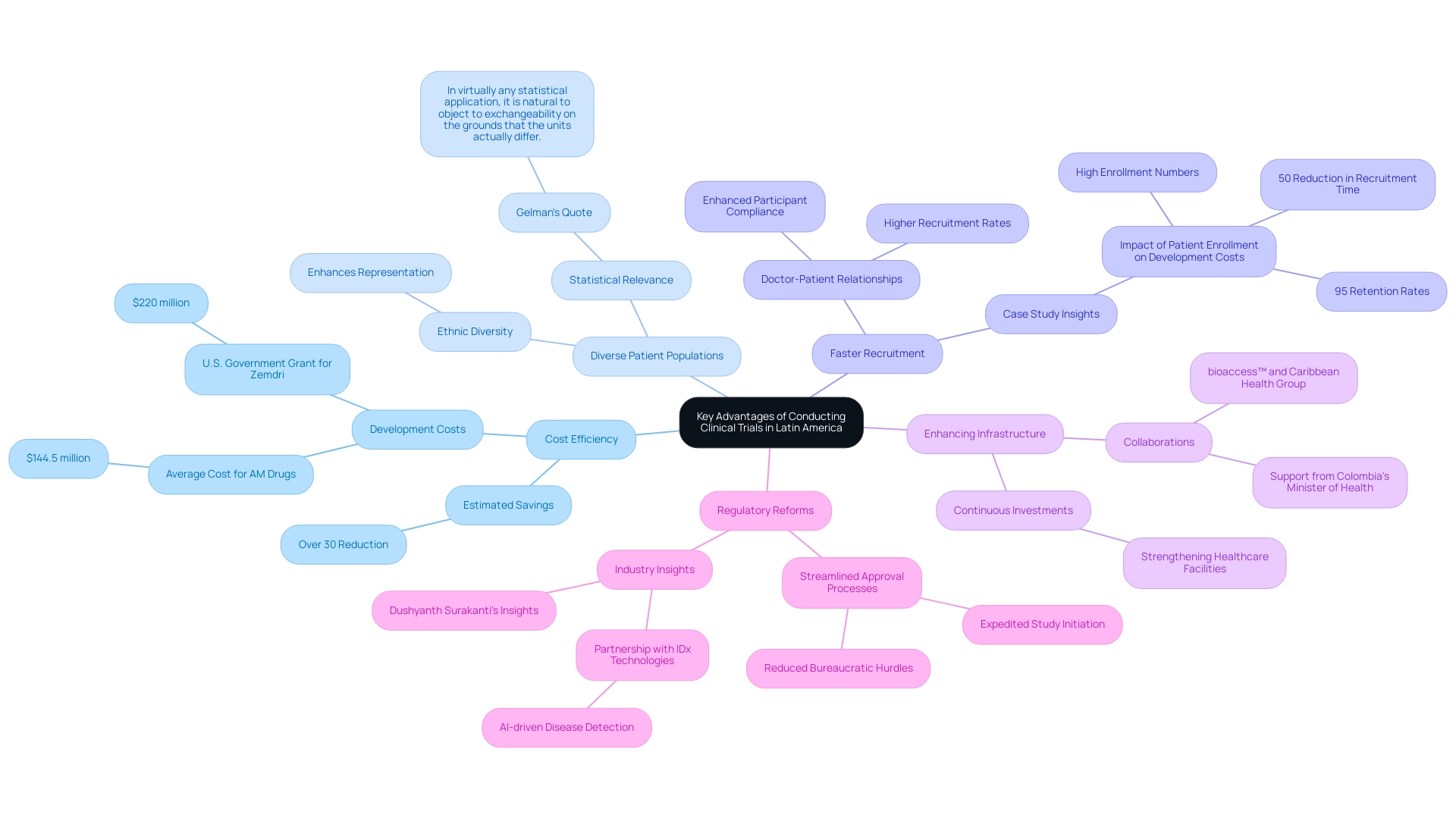
Challenges and Considerations in Latin American Clinical Trials
Conducting medical studies in Latin regions presents a distinct array of obstacles that can influence the overall research procedure. However, the clinical trial regulation benefits in Latin America can help mitigate some of these challenges.
- Regulatory Complexity: The approval process can be arduous due to varying regulations across countries, often leading to delays. Navigating these regulatory landscapes necessitates a profound understanding of local laws, which can differ significantly. This complexity is further compounded by the need for compliance reviews and managing interactions with local regulatory authorities, such as INVIMA, the Colombia National Food and Drug Surveillance Institute. INVIMA oversees medical device classification and compliance as a Level 4 health authority recognized by PAHO/WHO, illustrating the clinical trial regulation benefits in Latin America.
- Recruitment Issues: Although patient enthusiasm remains high, logistical challenges arising from differing healthcare systems can hinder recruitment efforts. For instance, while dropout rates in Latin America are notably lower—one-third of those in the U.S. and EU—recruitment remains a critical hurdle. Comprehensive research management services, including feasibility studies and site selection, are essential for overcoming obstacles related to clinical trial regulation benefits in Latin America.
- Infrastructure Gaps: Certain areas still face a deficiency in sufficient investigative infrastructure, adversely influencing data quality and management of experiments. Investment in facilities and resources is vital to support medical research effectively, as the clinical trial regulation benefits in Latin America underscore the importance of organizations like bioaccess® in facilitating these needs.
- Cultural Sensitivity: Researchers must be acutely aware of cultural differences and ethical considerations that vary by country. Tailored approaches to patient engagement are essential to navigate these complexities successfully, ensuring that the clinical trial regulation benefits in Latin America resonate with local populations and meet ethical standards.
- Economic Instability: Fluctuating economic conditions in the region can significantly impact the availability of funding and resources for research studies. This economic instability necessitates careful financial planning and may influence the overall success of clinical trial regulation benefits in Latin America. As Dr. Sergio Alvarado, a Clinical Experiment Manager focused on innovative medical studies, states, ‘The importance of adaptability and continuous learning for job seekers amidst hiring freezes encourages professionals to build diverse skill sets.’ This sentiment resonates strongly within the sector of health studies, where the ability to adapt is crucial for overcoming these challenges and ensuring successful experiment execution.
In conclusion, the clinical trial regulation benefits in Latin America are contributing to its emergence as a significant player in health studies, driven by investments in infrastructure and a focus on patient-centric practices. The region's dedication to tackling these challenges positions it favorably for future expansion in medical research, underscoring the clinical trial regulation benefits in Latin America, ultimately aiding local economies and health outcomes. Furthermore, effective study management requires a clear comprehension of the roles of principal investigators and the significance of site selection, compliance reviews, and reporting mechanisms for study status and adverse events, which are essential for preserving the integrity and success of research.
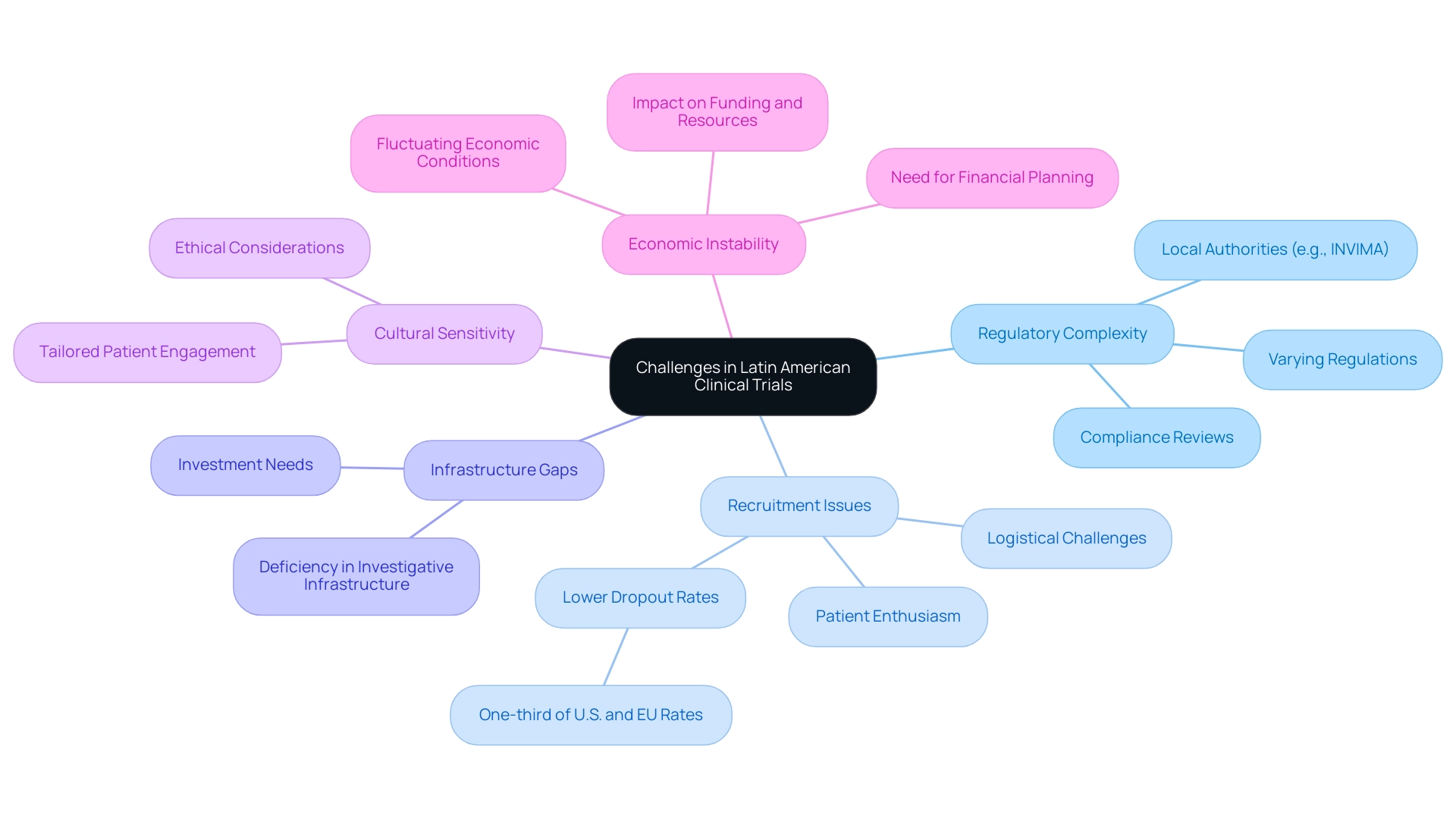
Cultural and Ethical Considerations in Clinical Research
When conducting clinical studies in Latin America, particularly in Colombia, it is essential to consider cultural and ethical aspects to fully understand the clinical trial regulation benefits in the region.
Informed Consent: Achieving a thorough understanding of the trial's objectives and methods among participants is critical. A recent study indicated that 18% of participants had not thoroughly read the study information letter, and alarmingly, the proportion of participants who understood informed consent had not increased over a 30-year period. This underscores the necessity for improved informed consent practices. Moreover, a concerning 10% admitted to fearing questions, highlighting significant gaps in communication and comprehension.
Community Engagement: Establishing trust within local communities is vital for enhancing participant recruitment and retention. Engaging community leaders and stakeholders can cultivate goodwill and ensure that the study aligns with local values and needs.
Respect for Local Customs: Ethical compliance is deeply rooted in understanding and respecting cultural practices unique to each community. This respect not only comforts participants but also strengthens the ethical foundation of the study.
Ethical Review: Adhering to local ethical guidelines and securing approvals from ethical committees, such as those overseen by INVIMA—the Colombia National Food and Drug Surveillance Institute—are paramount for maintaining research integrity. Roberto F. Iunes aptly stated,
That is why in order to achieve an equitable realization of the right to health for all, a multi-systemic approach is necessary,
emphasizing the need for comprehensive ethical oversight.
Diversity in Participation: Prioritizing varied representation in medical studies is essential for producing results that are applicable to diverse populations. This inclusion helps bridge knowledge gaps regarding new cancer treatments, ensuring that advancements in healthcare are accessible to all segments of society.
A pertinent case study titled 'Continuity of Care Post-Research Studies' discusses mechanisms to ensure ongoing care for individuals participating in research in Latin America, recommending a WHO resolution to ensure that sponsors provide ongoing care to prevent financial ruin for participants. Additionally, media coverage by Clinical Leader underscores the expansion of research in the region, highlighting the significance of grasping the regulatory environment and cultural subtleties for leveraging clinical trial regulation benefits in Latin America. This coverage not only informs stakeholders about the evolving landscape but also shapes perceptions and practices within research management services, reinforcing the need for adherence to ethical standards and community engagement.
The case study on continuity of care illustrates practical applications of these themes, demonstrating how ongoing support for participants can enhance trust and participation in future studies.
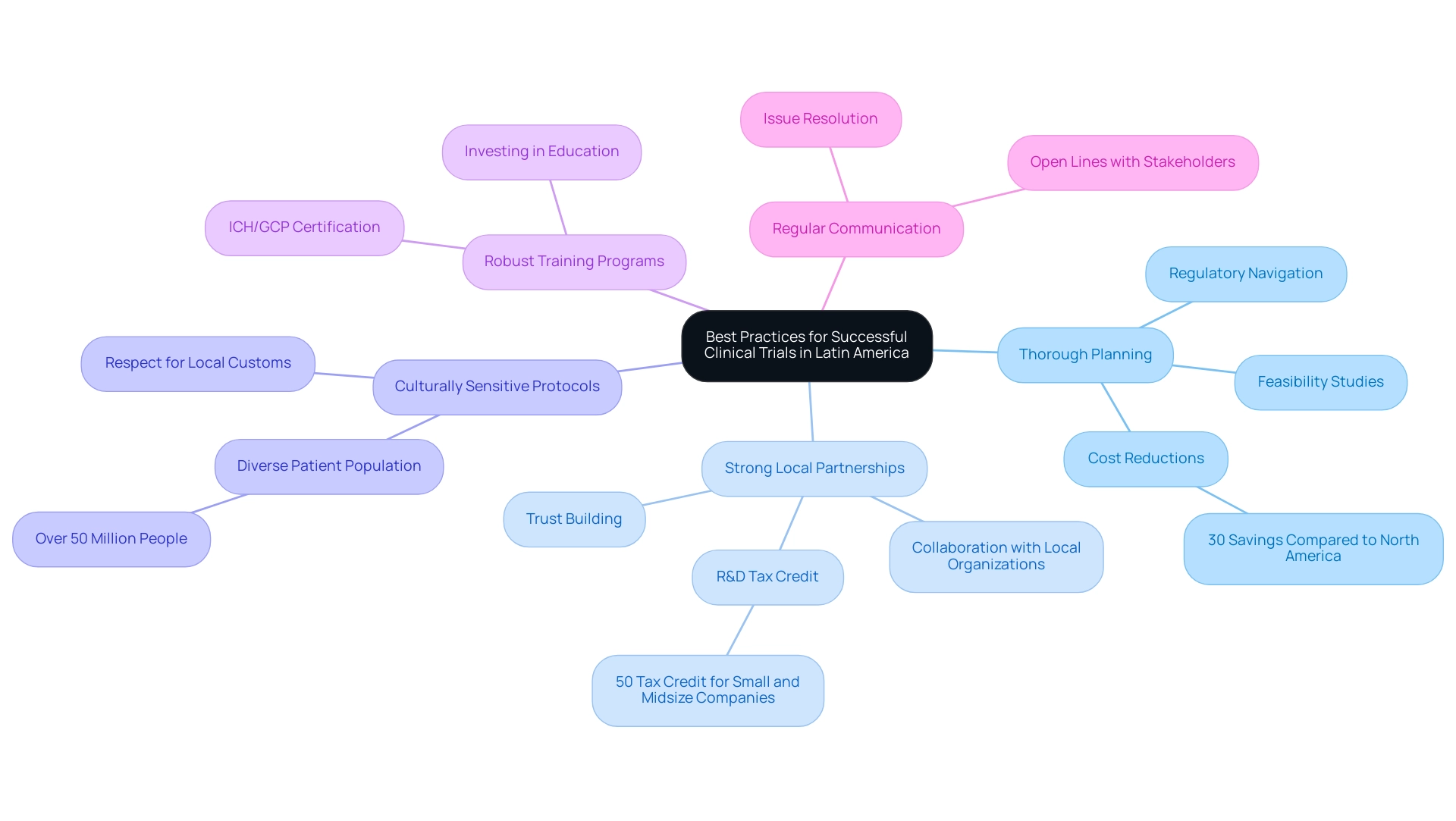
Best Practices for Successful Clinical Trials in Latin America
To optimize clinical study success in Latin America, particularly in Colombia, several best practices must be prioritized:
- Thorough Planning: Conducting feasibility studies is essential for navigating the local regulatory landscape and understanding patient demographics prior to initiating a study. This preliminary step is crucial for aligning study objectives with regional realities. In Colombia, the total IRB/EC and Ministry of Health (INVIMA) review processes are notably efficient, taking only 90 to 120 days. This efficiency exemplifies the clinical trial regulation benefits in Latin America by significantly enhancing study timelines. Furthermore, conducting experiments in Colombia can lead to cost reductions exceeding 30 percent compared to similar studies in North America or Western Europe, illustrating the economic appeal of clinical trials in the region.
- Strong Local Partnerships: Collaborating with local research organizations and healthcare providers is vital for enhancing understanding of the community. Julio G. Martinez-Clark, CEO of bioaccess, emphasizes that Colombia has recognized the clinical trial regulation benefits in Latin America and has developed an ambitious science, technology, and innovation plan for 2022–2031 to transition into a knowledge economy. Such partnerships not only facilitate recruitment but also foster trust among participants. With a universal healthcare system covering approximately 95% of the population, the clinical trial regulation benefits in Latin America underscore the importance of engaging local stakeholders for successful recruitment strategies. Additionally, the new Colombian R&D tax credit allows small and midsize companies to claim a 50% tax credit on their R&D and innovation projects, reflecting the clinical trial regulation benefits in Latin America by providing a strong financial incentive for collaboration.
- Culturally Sensitive Protocols: Developing study protocols that respect local customs and healthcare practices is crucial for ensuring participant comfort and compliance. This approach makes studies more relatable and accessible to the target population, further enhanced by Colombia's diverse and extensive patient population of over 50 million.
- Robust Training Programs: Comprehensive training for investigators and staff is necessary to uphold adherence to protocols and ethical standards. This investment in education is essential for maintaining the integrity of the process and enhancing participant safety. Given the rigorous ICH/GCP certification process that hospitals must undergo to conduct clinical research, the importance of training cannot be overstated.
- Regular Communication: Maintaining open lines of communication with all stakeholders is essential for addressing any issues promptly and facilitating smoother operations. Effective communication fosters a collaborative atmosphere that supports the success of the study.
Engaging local investigators and building trust with stakeholders significantly enhances study outcomes and participant satisfaction, thereby showcasing the clinical trial regulation benefits in Latin America and ultimately contributing to local economies and healthcare improvements. For instance, as highlighted in the case study titled 'Building Partnerships: Collaborating with Local Stakeholders,' strong partnerships with local stakeholders are critical for successful research in Latin regions. These collaborations enhance understanding of patient populations and improve recruitment strategies, demonstrating the clinical trial regulation benefits in Latin America through local engagement.
Moreover, Colombia's healthcare system is ranked 22nd by the World Health Organization, with its hospitals recognized as among the finest in Latin nations, further emphasizing the quality advantages of conducting studies in the country.
The Future of Clinical Trials: Trends and Predictions for Latin America
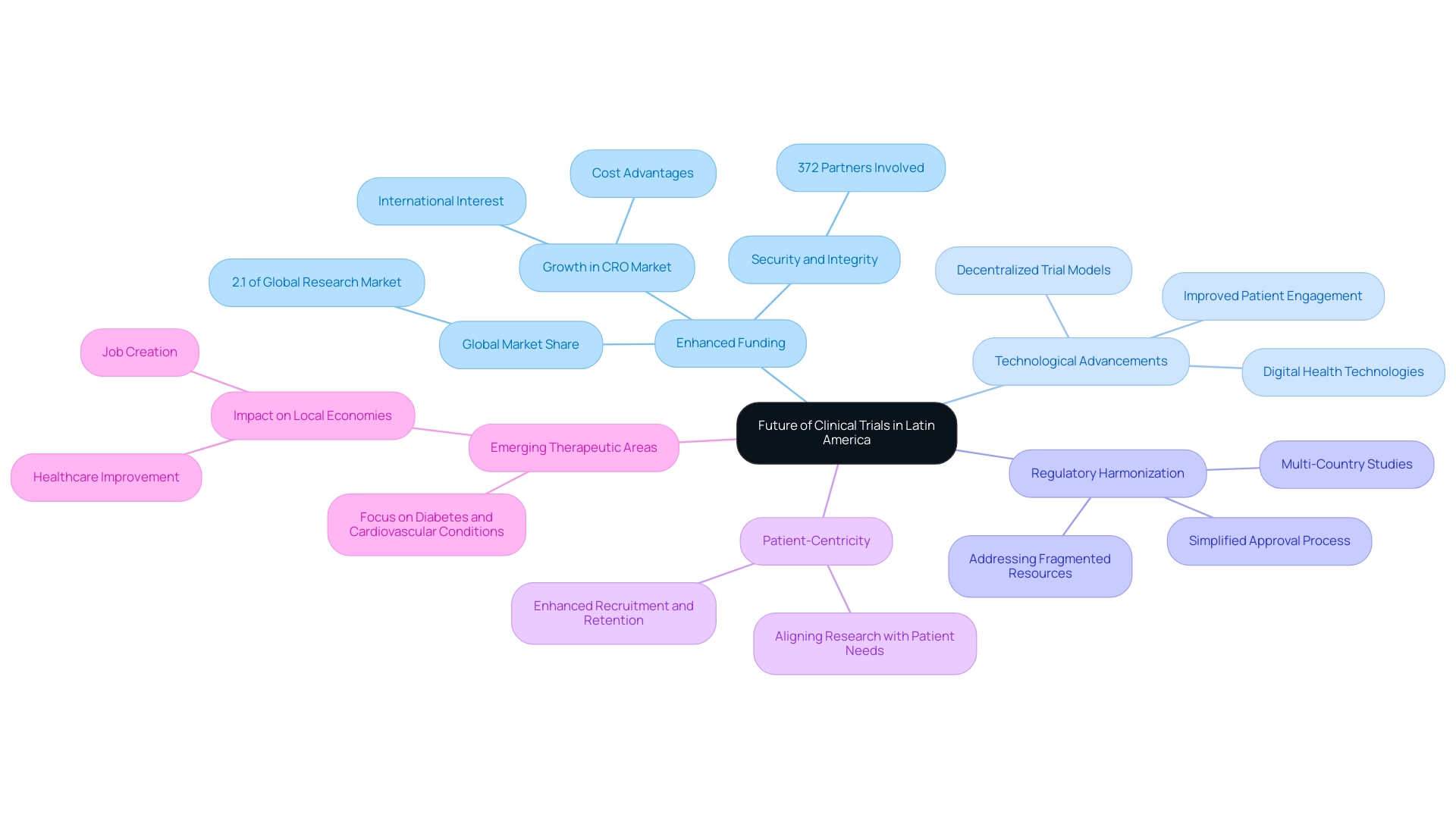
The perspective for medical studies in Latin regions is becoming increasingly favorable, characterized by several crucial trends that are shaping the environment:
- Enhanced Funding: With substantial advancements in research facilities and the backing of firms like bioaccess®, Latin America is emerging as a desirable location for sponsors seeking to invest in studies. A recent study indicates that the area represented 2.1% of the global research market in 2023. Projections suggest that Mexico and Brazil will experience significant growth in the Contract Research Organization (CRO) market, driven by their cost advantages and rising international interest. This growth is further supported by the involvement of 372 partners who ensure security, prevent and detect fraud, and rectify errors, thereby enhancing the integrity of trials.
- Technological Advancements: The integration of digital health technologies and decentralized trial models is on the rise, promising to improve patient engagement and streamline data collection. This shift is not merely a response to current demands but a proactive step toward enhancing the efficiency and effectiveness of clinical trials.
- Efforts aimed at harmonizing regulations across various countries in Latin America are expected to simplify the approval process, thereby highlighting the benefits of clinical trial regulations in the region. This regulatory alignment will facilitate the execution of multi-country studies and emphasize the advantages of conducting clinical trials in Latin America, making it easier for sponsors to navigate the complexities of investigations across different jurisdictions. Addressing fragmented resources, language barriers, and the lack of CRO corporate structures highlighted in the Medtech landscape is crucial for unlocking the region's potential.
- Focus on Patient-Centricity: A notable shift toward patient-centric study designs is emerging, underscoring the importance of aligning research with the needs and preferences of patients. This focus is anticipated to enhance recruitment and retention rates, ultimately leading to more successful study outcomes.
- Emerging Therapeutic Areas: As healthcare challenges evolve, there is a growing emphasis on trials targeting diseases prevalent in the region, such as diabetes and cardiovascular conditions. This shift not only addresses pressing health concerns but also aligns with global research priorities. The impact of Medtech research studies on local economies is significant, contributing to job creation, economic growth, and healthcare improvement.
As Gotuzzo from Universidad Peruana Cayetano Heredia aptly states, > Research in Latin America: Constraints and Opportunities. The region is poised for a transformative phase that will leverage these trends to enhance the quality and influence of research studies, fueled by cooperation and a dedication to innovation and regulatory excellence. Comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential to address the challenges faced by Medtech companies and ensure successful outcomes in this rapidly evolving landscape.
Conclusion
The regulatory landscape for clinical trials in Latin America, particularly in Colombia, presents a wealth of opportunities for researchers aiming to conduct innovative studies. With significant cost savings, rapid approval processes, and a robust healthcare system, Colombia emerges as a prime destination for clinical research. The ongoing reforms aimed at streamlining regulations further enhance the region's appeal, creating a favorable environment for both local and international stakeholders.
However, despite these advantages, navigating the complexities of varying regulations, cultural sensitivities, and infrastructure gaps poses challenges that must be addressed. Understanding the local regulatory frameworks and engaging with communities are essential strategies for successful trial execution. By prioritizing ethical considerations and cultural awareness, researchers can foster trust and enhance participant recruitment and retention.
Looking ahead, Latin America is poised for growth in clinical research, driven by increased investment, technological advancements, and a focus on patient-centricity. The collaboration between local stakeholders and the commitment to regulatory harmonization will play a crucial role in unlocking the region's full potential. As the landscape continues to evolve, the dedication to high-quality research and ethical standards will ensure that Latin America remains a vital player in the global clinical trial arena.
Frequently Asked Questions
What are the key regulatory benefits for conducting clinical trials in Latin America?
Key regulatory benefits include cost efficiency with savings over 30% compared to North America and Europe, diverse patient populations for better representation, faster recruitment rates due to strong doctor-patient relationships, enhancing infrastructure through continuous investments, and recent regulatory reforms that streamline approval processes.
How does Colombia's regulatory framework support first-in-human studies?
Colombia offers competitive advantages such as cost reductions exceeding 30% compared to North America and Western Europe, along with a swift approval process where IRB/EC and INVIMA assessments are completed within 90-120 days.
What steps must researchers take to comply with Colombian regulations?
Researchers must familiarize themselves with INVIMA guidelines and processes, including obtaining IRB/EC approval, INVIMA approval, and the MinCIT import permit.
What is the significance of Colombia's healthcare system for clinical trials?
Colombia's healthcare system is ranked among the top five globally, and with over 50 million individuals and 95% covered by universal healthcare, it presents a significant patient recruitment potential for clinical trials.
What recent reforms are enhancing clinical trial regulations in Latin America?
Recent reforms include Brazil allowing local ethics committees to independently authorize research protocols, thus expediting the approval process for multi-site studies, and Colombia's ambition to develop a knowledge-driven economy by 2031.
How do R&D tax incentives impact clinical trials in Colombia?
R&D tax incentives, including generous tax deductions and credits, facilitate growth by streamlining approval processes and harmonizing guidelines for clinical trials.
Why is Latin America's ethnic diversity important for clinical research?
The region's rich ethnic diversity enhances the representation of various demographics in research studies, which is essential for accurately assessing drug efficacy and safety across different populations.
What role does patient recruitment play in the success of clinical trials in Latin America?
High recruitment rates and participant compliance, aided by robust doctor-patient relationships, ensure studies progress swiftly and can significantly influence development costs.
How are infrastructure investments affecting medical studies in Latin America?
Continuous investments in healthcare and research facilities strengthen the capacity to conduct high-quality medical studies, vital for meeting international standards.
What certification must hospitals in Colombia achieve to conduct research with pharmaceutical drugs?
Hospitals must pass a rigorous ICH/GCP certification process to ensure high standards of quality and safety before conducting research with pharmaceutical drugs.




