Overview
The article focuses on understanding the evolving landscape of clinical trials in Latin America, highlighting key advantages, challenges, and future trends for researchers in the region. It emphasizes that Latin America presents a unique opportunity for clinical trials due to cost efficiency, diverse patient populations, and faster recruitment, while also addressing regulatory complexities and cultural considerations that researchers must navigate to successfully conduct studies.
Introduction
The clinical trial landscape in Latin America is experiencing a remarkable evolution, marked by a surge in registrations and an increasing interest from global sponsors. As lower middle-income countries witness a significant uptick in trial activity, the region is becoming an attractive focal point for conducting clinical research.
Factors contributing to this trend include:
- Diverse patient populations
- Cost efficiencies
- Enhanced regulatory frameworks
These elements position Latin America as a compelling destination for innovative medical studies. However, navigating the complexities of this landscape requires a thorough understanding of the local regulatory environment, cultural nuances, and the unique challenges that accompany clinical trials in this vibrant region.
This article delves into the key advantages, obstacles, and future trends shaping clinical trials in Latin America, offering insights into how stakeholders can effectively engage with this dynamic market.
The Evolving Landscape of Clinical Trials in Latin America
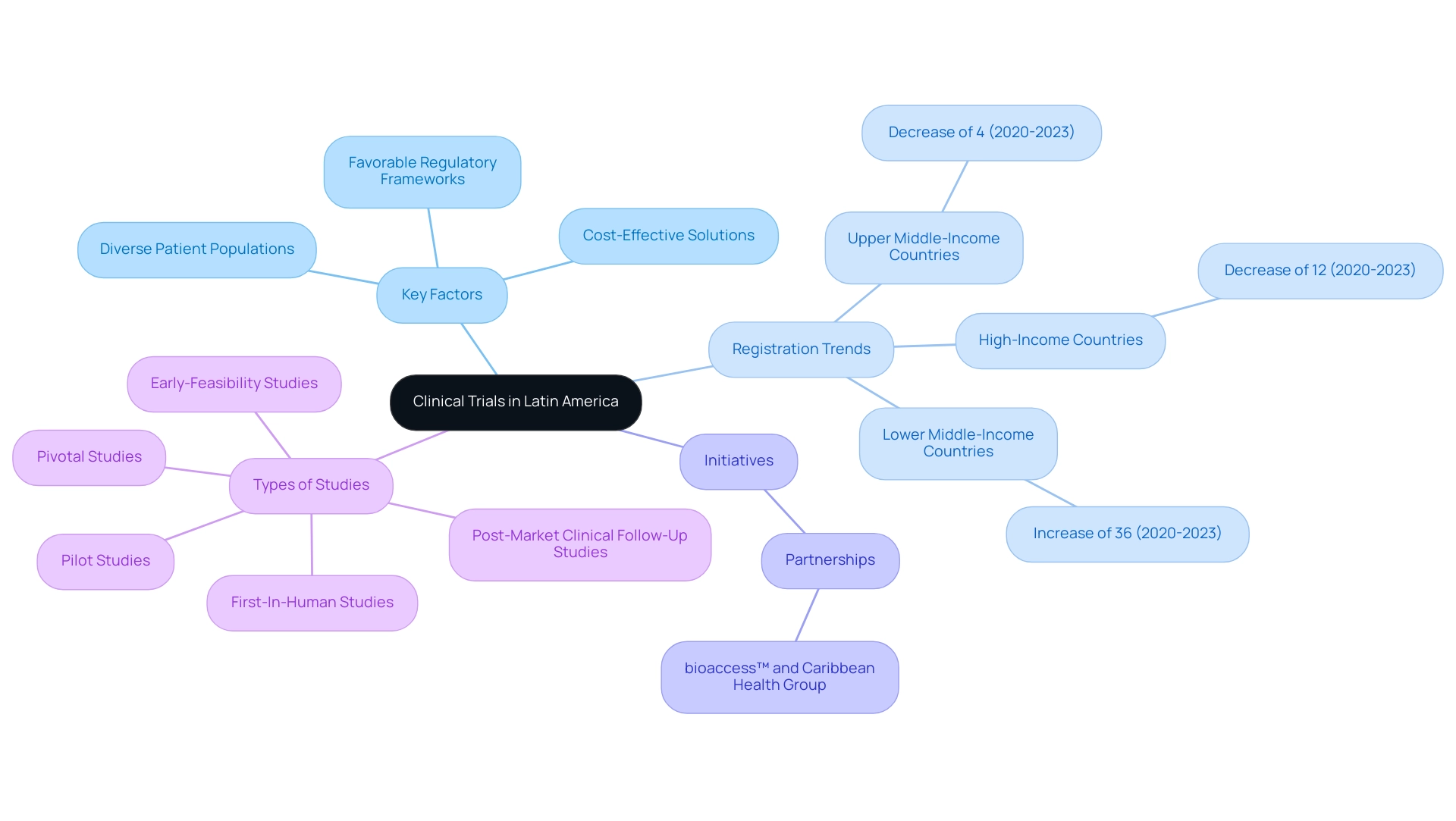
Latin America has emerged as an appealing location for clinical trials in Latin America, driven by several key factors including its diverse patient populations, favorable regulatory frameworks, and cost-effective solutions. Recent trends indicate that while upper middle-income and high-income nations saw a decline in registrations of 4% and 12% respectively from 2020 to 2023, lower middle-income countries experienced a remarkable 36% increase in registrations during the same period. This growth underscores the region’s potential for strong medical studies, illustrated by initiatives such as the partnership between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, aimed at establishing Barranquilla as the premier location for medical evaluations in Latin America, with assistance from Colombia's Minister of Health.
Furthermore, Argentina is expected to record the highest compound annual growth rate (CAGR) from 2024 to 2030, enhancing its appeal to trial sponsors. The growing number of trial organizations (CROs) establishing operations in Latin America highlights a commitment to conducting clinical trials in Latin America while fulfilling international standards and addressing local needs. These CROs, including partnerships with organizations like GlobalCare Clinical Trials, are enhancing capacity for swift participant recruitment—achieving over 50% reductions in recruitment time and 95% retention rates—while fostering collaborations with local institutions to tailor their approaches to the unique public health challenges of the region.
Additionally, bioaccess® specializes in various types of studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This collaborative spirit is vital for advancing medical research and addressing the diverse health needs of the population.
Key Advantages of Conducting Clinical Trials in Latin America
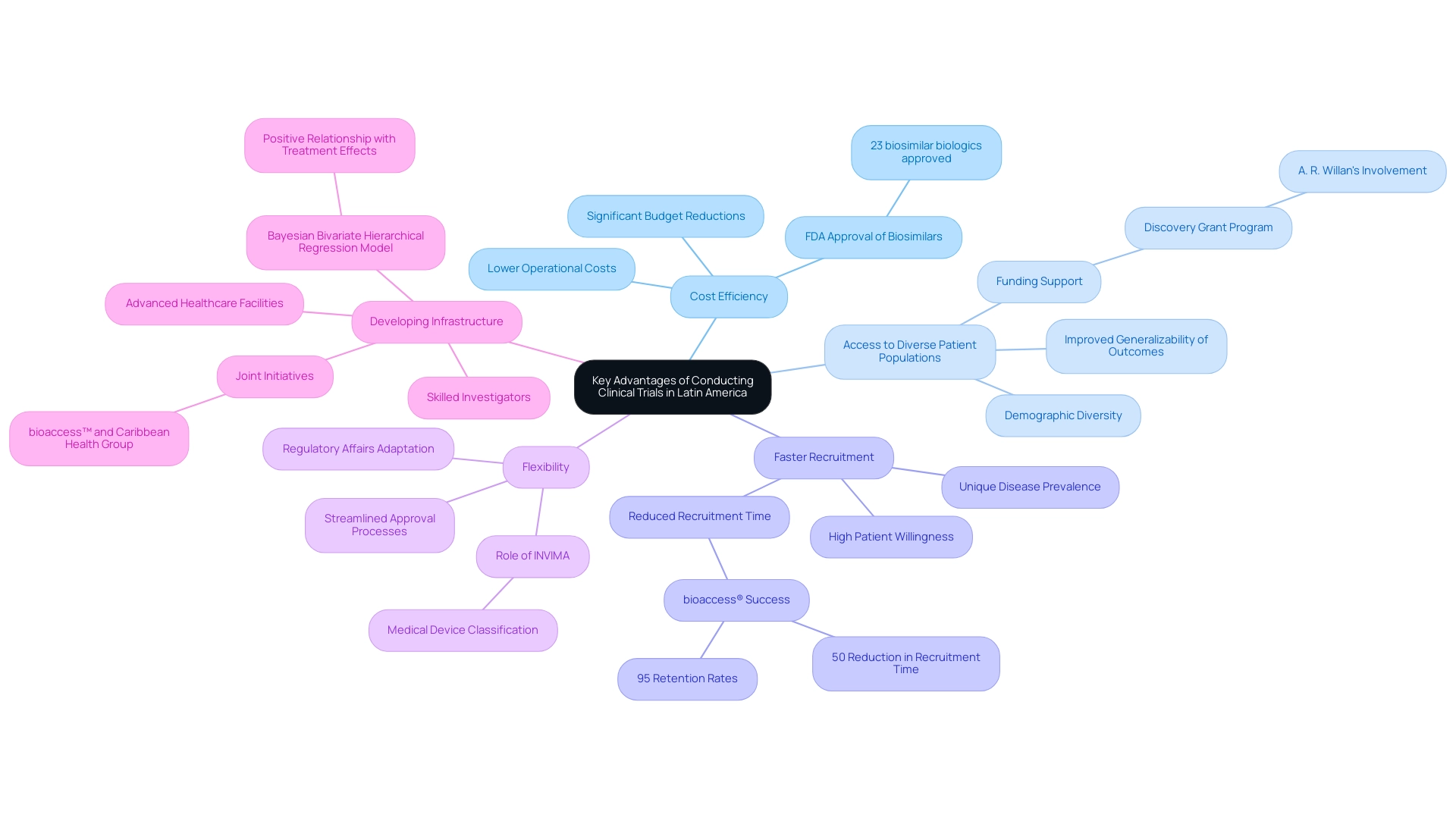
- Cost Efficiency: Carrying out research studies in Latin America offers a significant cost benefit in comparison to North America and Europe. The region benefits from significantly lower operational costs, including reduced labor and facility expenses, leading to a substantial decrease in the overall budget of a study. This financial advantage is crucial, particularly in a time when optimizing resources is vital for successful execution. As demonstrated by recent patterns, the cost-effectiveness of clinical trials in Latin America continues to draw interest from sponsors seeking to enhance their investment. In fact, as of October 2019, the FDA had approved 23 biosimilar biologics for 9 reference products, highlighting the growing significance of cost-effective studies in the regulatory landscape.
- Access to Diverse Patient Populations: Latin America is characterized by its rich demographic diversity, providing researchers access to various patient populations. This heterogeneity is vital for investigating diseases that present differently across ethnicities in the context of clinical trials in Latin America, allowing for a more nuanced understanding of treatment effects. Furthermore, varied patient inclusion improves the generalizability of study outcomes, consistent with suggestions from specialists who emphasize the significance of demographic diversity in medical investigations. As A. R. Willan observes, funding through programs like the Discovery Grant can significantly enhance credibility of studies and support diverse patient involvement in trials.
- Faster Recruitment: The region’s unique disease prevalence and the high willingness of patients to engage in research studies contribute to accelerated recruitment timelines. This efficiency not only shortens the duration of studies but also enables quicker access to vital data, facilitating the advancement of medical research. As sponsors increasingly recognize these advantages, they find that clinical trials in Latin America become an attractive option for timely study completions. Notably, bioaccess® has demonstrated success in this area, achieving over 50% reduction in recruitment time and 95% retention rates through its strategic partnerships and expertise in clinical trial management.
- Flexibility: Many Latin American nations have made significant progress in refining their frameworks, resulting in more streamlined approval processes. This oversight flexibility allows researchers to navigate the complexities of initiating studies with greater ease, expediting the overall timeline from concept to execution. The changing environment of Regulatory Affairs in the area corresponds effectively with the increasing need for efficient trial methodologies. INVIMA, as Colombia's National Food and Drug Surveillance Institute, plays a crucial role in this space, overseeing medical device classification and ensuring compliance with international standards.
- Developing Infrastructure: The medical investigation framework in Latin America is swiftly progressing, with clinical trials in Latin America being marked by a rising number of skilled investigators, cutting-edge healthcare facilities, and committed study organizations. This development not only aids in a more effective evaluation process but also boosts the credibility and reliability of findings in the region. A recent case study involving a Bayesian Bivariate Hierarchical Regression Model demonstrated a positive relationship between treatment effects and country-specific covariates, reinforcing the need to account for demographic diversity in medical studies. Joint initiatives, like those between bioaccess™ and Caribbean Health Group, are additionally establishing Colombia, especially Barranquilla, as a significant participant in global medical research, highlighting its potential for clinical trials in Latin America. bioaccess® specializes in managing a variety of research studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), ensuring a comprehensive approach to study management.
Challenges and Considerations for Clinical Trials in Latin America
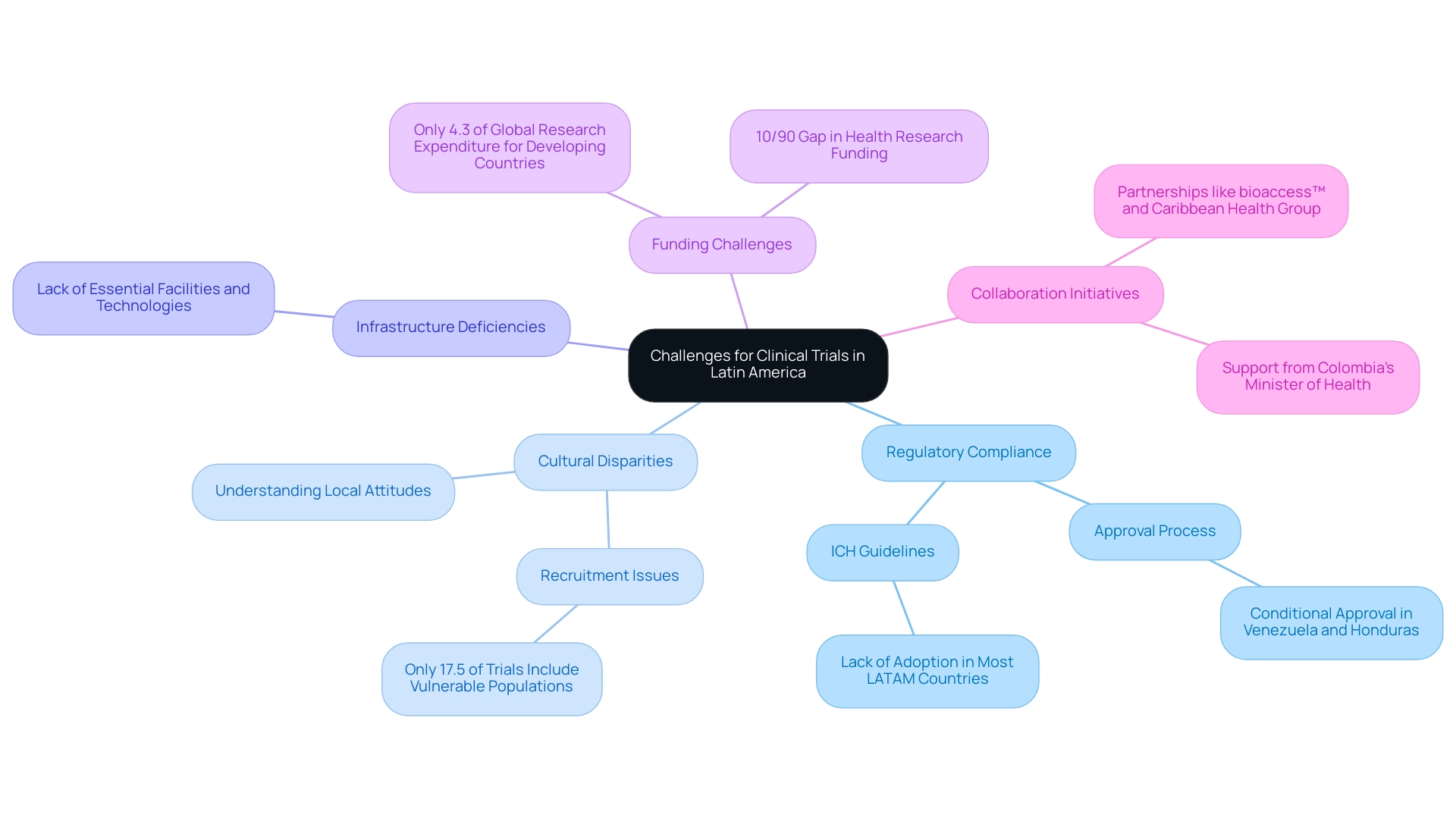
Navigating the terrain of medical study compliance presents significant challenges for clinical trials in Latin America due to the unique guidelines in each nation, complicating the approval process for multinational research. For instance, Venezuela and Honduras uniquely accept conditional approval for drugs deemed especially necessary, highlighting the regulatory divergence that exists across the region. Numerous Latin American nations have not yet conformed to the International Council for Harmonization (ICH) guidelines, which restricts the adoption of revised regulations, creating additional challenges for researchers who must maneuver through local laws and requirements.
Cultural disparities additionally affect the effectiveness of studies in the region. It is essential for researchers to comprehend the attitudes and beliefs related to research, as misalignment can lead to recruitment difficulties and impede participant retention. A case study focused on the recruitment of vulnerable populations, such as children and pregnant women, revealed that only 17.5% of studies included these groups, underscoring a significant gap in inclusivity and cultural understanding.
This emphasizes the necessity for thorough feasibility studies and compliance evaluations to ensure that clinical studies are designed with cultural sensitivities considered. Additionally, infrastructure deficiencies exist in some regions, where essential facilities and technologies required for advanced clinical trials in Latin America are missing, which can hinder execution and impact data quality. Communication barriers, stemming from language differences and varying levels of expertise among local staff, can further complicate matters, potentially leading to misunderstandings that impact study outcomes.
Additionally, funding challenges are significant, as only 4.3% of global research expenditure is allocated to address the health needs of developing countries, reflecting the ongoing 10/90 gap in health research funding. Economic fluctuations and political instability in certain nations can present additional risks, impacting the feasibility and safety of carrying out tests. As highlighted by industry specialists like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, a professional in regulatory matters for medical devices in Colombia, understanding these dynamics is crucial for researchers conducting clinical trials in Latin America to effectively maneuver through the intricate environment of Regulatory Affairs.
Collaboration initiatives, such as the one between bioaccess™ and Caribbean Health Group, seek to establish Barranquilla as a prominent location for health studies in the region, backed by the approval of Colombia's Minister of Health. These efforts include comprehensive project management and reporting services to ensure that assessments meet both local and international standards.
Navigating Regulatory and Ethical Frameworks in Latin American Clinical Trials
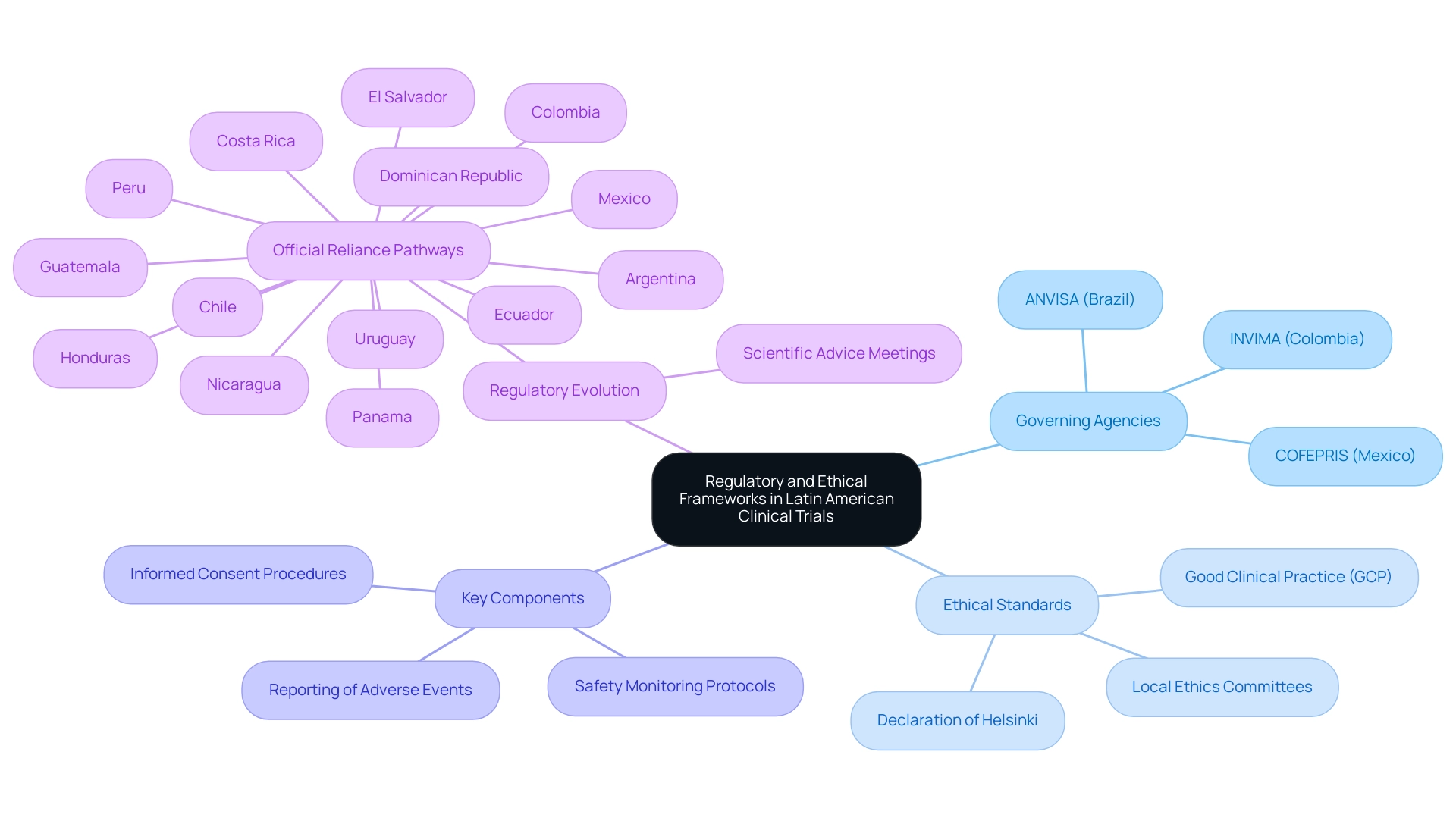
In traversing the terrain of clinical trials in Latin America, it is essential for researchers to be well-informed about the governing agencies supervising these studies, such as ANVISA in Brazil and COFEPRIS in Mexico. Each nation has developed its own regulatory framework for clinical trials in Latin America, stipulating specific guidelines that include critical components such as:
- Informed consent procedures
- Safety monitoring protocols
- Reporting of adverse events
Adherence to ethical standards is fundamental; thus, compliance with Good Clinical Practice (GCP) is non-negotiable.
To ensure that investigative activities meet local ethical expectations, early engagement with local ethics committees is recommended. Moreover, becoming acquainted with the Declaration of Helsinki and other relevant international guidelines is crucial for upholding the highest ethical standards throughout the process. As regulations evolve, particularly with the presence of official reliance pathways in countries including Argentina, Chile, Colombia, Costa Rica, and others, staying informed about these changes is crucial for researchers conducting clinical trials in Latin America who aim to facilitate timely access to innovative therapies for patients.
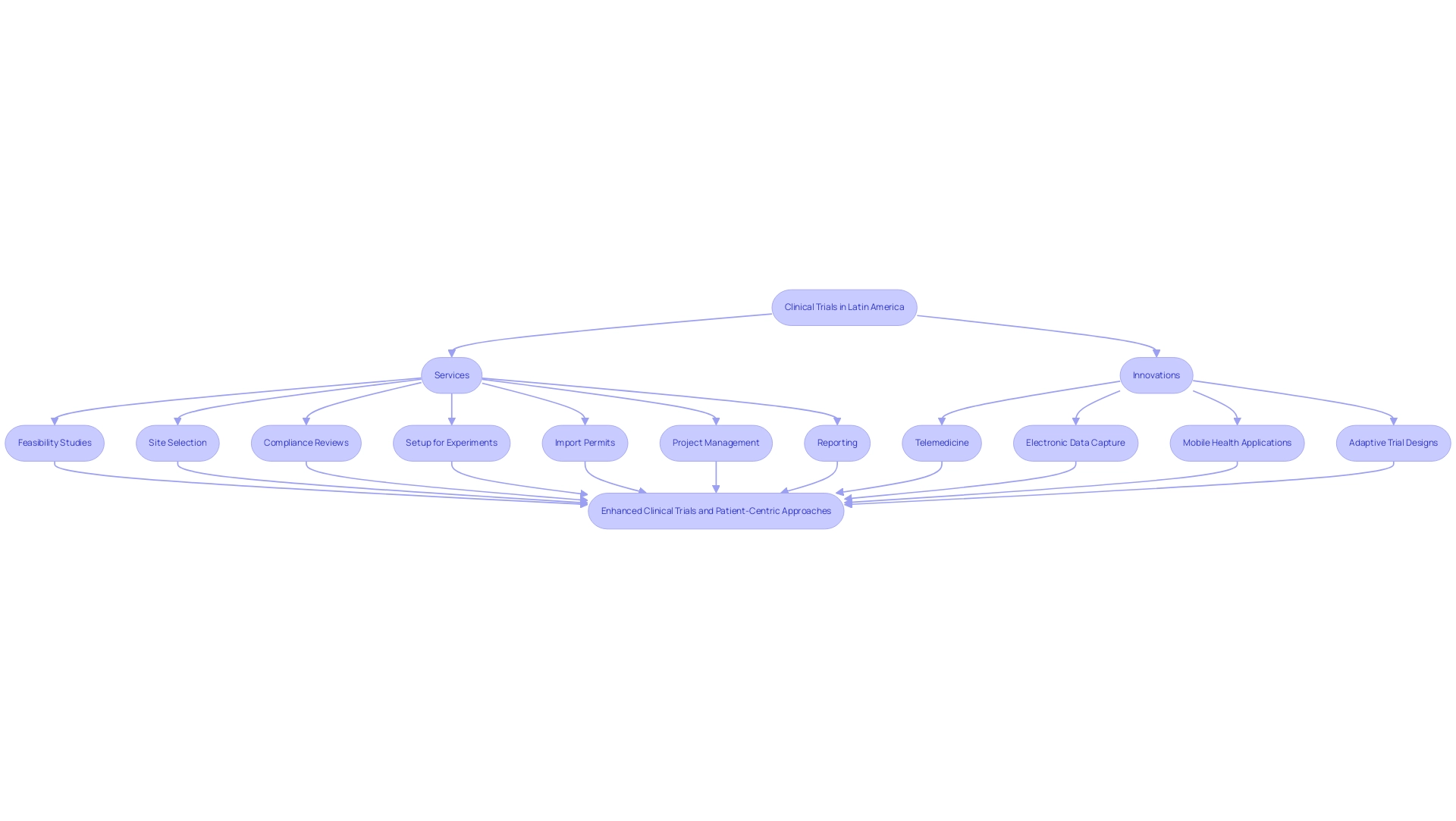
Our comprehensive clinical trial management services encompass:
- Detailed feasibility studies
- Meticulous site selection
- Thorough compliance reviews
- Efficient trial setup
- Management of import permits
- Proactive project management
We also provide extensive reporting on study status, inventory, and both serious and non-serious adverse events to streamline your research efforts. Additionally, Katherine Ruiz brings expert insight into Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, guiding researchers through INVIMA's regulatory functions and oversight as a Level 4 health authority by PAHO/WHO.
INVIMA plays a critical role in ensuring that clinical trials in Latin America comply with national regulations, impacting everything from the approval process to the monitoring of investigational devices. Furthermore, Virginia Cozzi highlights the significance of accountability in research, stating, 'Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.'
The case study on Scientific Advice Meetings highlights that while these meetings are common in reference regulations, they are only present in a few LATAM countries, indicating a gap in oversight support that researchers must navigate.
Future Trends and Innovations in Latin American Clinical Trials
The scenery of medical studies in Latin America, including clinical trials in Latin America, is experiencing a notable change, propelled by the growing application of technology and creative governance methods. Medtech firms encounter various obstacles, such as regulatory hurdles, language barriers, and fragmentation of resources, which bioaccess® tackles through its extensive management services for research. These services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup for experiments
- Import permits
- Project management
- Reporting
These services are specifically designed to facilitate seamless communication and collaboration between Latin American hospitals and US research clients.
Innovations such as telemedicine, electronic data capture, and mobile health applications are becoming essential to research studies, enhancing patient involvement and improving the efficiency of data collection processes. These advancements not only streamline operations but also promote a more inclusive environment for participants, thereby bridging gaps in medical research and innovation. As the focus shifts towards patient-centric approaches, clinical study designs are increasingly prioritizing the patient experience, incorporating flexible designs and encouraging greater patient involvement in planning stages.
Such strategies are essential for ensuring that experiments meet participants' needs, ultimately leading to more successful outcomes. Moreover, the rise of adaptive trial designs allows researchers to modify studies based on interim results, significantly enhancing efficiency and success rates. Collaboration and partnerships are anticipated to thrive, as global sponsors interact more with local institutions, a sentiment echoed by Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, who highlights the significance of a local presence in enhancing relationships with local talent and governing authorities.
On the governance front, Latin American countries are aligning their frameworks with global standards, exemplified by Costa Rica's commendable score in computational capacity and Uruguay's pioneering cannabis licensing framework. This regulatory advancement, coupled with technological integration, positions the region for clinical trials in Latin America, driving economic growth and healthcare improvements.
Conclusion
The evolution of clinical trials in Latin America presents a unique opportunity for stakeholders to leverage the region's diverse patient populations, cost efficiencies, and robust regulatory frameworks. With a remarkable increase in trial registrations, particularly in lower middle-income countries, Latin America is becoming a focal point for innovative medical research. The advantages of conducting clinical trials here, including faster recruitment times and a growing infrastructure, position the region favorably against traditional markets.
However, stakeholders must navigate a complex landscape marked by distinct regulatory challenges and cultural considerations. Understanding the local regulatory environments and the ethical implications of clinical research is essential for success. The ongoing efforts to enhance collaboration between local organizations and international sponsors will further streamline processes and address the specific health needs of the population.
Looking ahead, the integration of technology and patient-centric approaches will continue to transform the clinical trial landscape in Latin America. Innovations such as telemedicine and adaptive trial designs are not only improving efficiency but also fostering greater patient engagement. As the region aligns its regulatory frameworks with global standards, it is poised to become a leading destination for clinical research. Embracing these trends will be vital for stakeholders aiming to capitalize on the vast potential that Latin America offers for the future of clinical trials.
Frequently Asked Questions
Why has Latin America become an appealing location for clinical trials?
Latin America has emerged as an attractive site for clinical trials due to its diverse patient populations, favorable regulatory frameworks, and cost-effective solutions.
What recent trends have been observed in clinical trial registrations in Latin America?
From 2020 to 2023, lower middle-income countries in Latin America experienced a 36% increase in clinical trial registrations, while upper middle-income and high-income nations saw declines of 4% and 12%, respectively.
What initiatives are being undertaken to enhance clinical trials in Latin America?
Initiatives such as the partnership between bioaccess™ and Caribbean Health Group aim to establish Barranquilla, Colombia, as a premier location for medical evaluations, supported by Colombia's Minister of Health.
Which country in Latin America is expected to have the highest growth in clinical trials from 2024 to 2030?
Argentina is expected to record the highest compound annual growth rate (CAGR) from 2024 to 2030, increasing its appeal to trial sponsors.
How are Clinical Research Organizations (CROs) contributing to clinical trials in Latin America?
CROs are establishing operations in the region, enhancing capacity for participant recruitment, achieving over 50% reductions in recruitment time, and maintaining 95% retention rates while addressing local public health challenges.
What types of studies does bioaccess® specialize in?
bioaccess® specializes in various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
What are the cost advantages of conducting clinical trials in Latin America?
Research studies in Latin America offer significant cost benefits compared to North America and Europe, with lower operational costs leading to reduced overall study budgets.
How does demographic diversity in Latin America benefit clinical trials?
The region's rich demographic diversity allows researchers access to various patient populations, which is crucial for understanding disease variations and improving the generalizability of study outcomes.
What factors contribute to faster recruitment in Latin America?
Unique disease prevalence and a high willingness of patients to participate in research studies contribute to accelerated recruitment timelines, facilitating quicker access to vital data.
How have regulatory frameworks in Latin America improved for clinical trials?
Many Latin American nations have streamlined approval processes, allowing researchers to navigate study initiation complexities more easily and expediting timelines from concept to execution.




