Overview
The article addresses the critical phases of medical device clinical trials, underscoring their essential role in ensuring device safety and efficacy before market introduction. It delineates a structured progression through various trial stages—such as Early-Feasibility Studies and Post-Market Surveillance—while emphasizing the necessity for compliance with regulatory standards. Furthermore, it highlights the influence of demographic factors on trial outcomes, thereby reinforcing the trials' significance in fostering innovation and upholding patient safety.
Introduction
In the realm of healthcare innovation, medical device clinical trials serve as a crucial pillar, ensuring that new technologies are both safe and effective prior to their market introduction. These structured investigations not only assist manufacturers in navigating complex regulatory landscapes but also instill confidence in healthcare providers and patients alike.
As the industry evolves, grasping the multifaceted nature of these trials—from initial feasibility studies to post-market surveillance—becomes increasingly essential.
With a focus on emerging trends, regulatory challenges, and the significant role of demographic considerations, this exploration delves into the intricate journey of medical devices through clinical trials. It highlights the importance of strategic planning and expert guidance in advancing medical technology.
Understanding Medical Device Clinical Trials: An Overview
Medical equipment research studies represent organized examinations aimed at assessing the safety and efficacy of novel medical instruments. These assessments are crucial in ensuring that products comply with standards before their introduction to the market. The phases of medical device clinical trials typically encompass multiple stages, each designed to achieve specific objectives, ranging from initial safety assessments to extensive efficacy studies.
In 2025, the significance of trials in the medical device sector cannot be overstated. They are essential not only for regulatory approval but also for instilling confidence among healthcare providers and patients. Recent findings indicate that patients aged 45-49 years exhibited a significantly higher likelihood of achieving improved near vision (88%) compared to those aged 55-60 years (78%) after one year of treatment with the KAMRA corneal implant.
This underscores the importance of demographic factors in medical evaluations, as younger patients tend to experience better outcomes. The KAMRA study highlights the necessity of subgroup analyses in medical studies. As Diana M Zuckerman from the National Center for Health Research noted, "Despite the FDA's statements that such subgroup analyses are very important, only a small percentage of public reviews or official device labeling included information about these analyses, even when they were available in documents provided to the Advisory Committees."
As the Medtech environment evolves, current trends suggest an increasing focus on the clarity of research data. However, concerns arise that mandatory disclosure requirements may compel manufacturers to conduct investigations offshore to protect confidentiality. This potential shift could impact the accessibility of essential information that informs both oversight decisions and medical practice.
At bioaccess®, we specialize in extensive research study management services tailored to the specific needs of the medical apparatus sector in Latin America. Our expertise encompasses Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. We understand the governance landscape, including the role of INVIMA as Colombia's Level 4 health authority, ensuring compliance and oversight throughout the testing process.
In summary, the phases of medical device clinical trials are vital for the advancement of medical devices, ensuring their safety and effectiveness for public use. They not only facilitate regulatory approval but also enhance the credibility of manufacturers in a competitive market. As the sector continues to innovate, staying informed about these advancements and understanding the nuances of study design will be essential for stakeholders aiming to navigate the complexities of bringing groundbreaking medical technologies to fruition.
Classification of Medical Devices: Implications for Clinical Trials
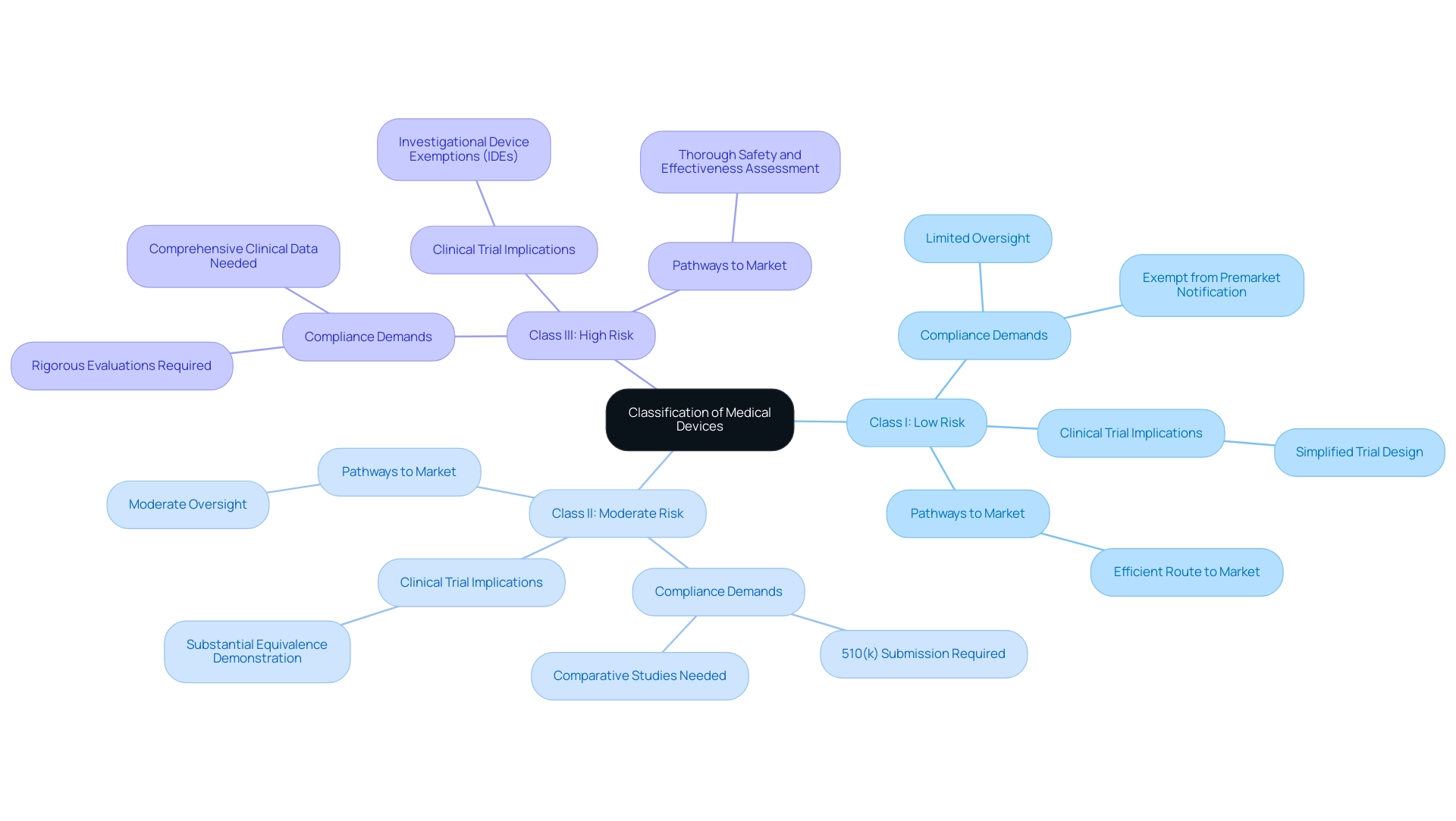
Medical instruments are categorized into three primary classes: Class I (low risk), Class II (moderate risk), and Class III (high risk). Each category entails specific compliance demands that significantly influence the research procedures necessary for gaining approval. For instance, Class I products generally undergo limited oversight, often exempt from premarket notification, facilitating a more efficient route to market.
Conversely, Class III products, which pose the highest risk, are subjected to rigorous evaluations designed to thoroughly assess their safety and effectiveness prior to market entry.
Understanding these classifications is crucial for manufacturers, particularly for medical technology startups, as it directly impacts their research strategies. The implications of product classification extend beyond mere regulatory compliance; they also affect the phases of medical device clinical trials, including trial design, patient recruitment, and overall study timelines. For example, Class II devices typically require a 510(k) submission, which necessitates comparative studies to demonstrate substantial equivalence to existing devices, while Class III devices must provide comprehensive clinical data through Investigational Device Exemptions (IDEs).
Recent statistics indicate that as of 2025, the oversight landscape for medical devices is evolving, with an increasing emphasis on risk management and post-market surveillance. This shift is particularly pertinent for advanced therapies, such as gene editing and mRNA technologies, which necessitate adaptive governance frameworks that balance innovation with patient safety. Case studies focusing on advanced therapies and governance frameworks illustrate how oversight bodies are updating guidelines to accommodate these complexities, fostering an environment where innovation can flourish while ensuring the safety and efficacy of new therapies.
Moreover, expert opinions underscore the necessity for a streamlined approach to oversight requirements that supports innovation without compromising safety. Notably, there was no consensus among experts regarding alternative regulatory approaches, such as the FDA's certification of software companies based on their quality-control systems. As the medical equipment sector advances, understanding the nuances of classification and its implications for research studies will be vital for successful product development and market entry.
At bioaccess®, we offer comprehensive management services for research studies designed to navigate these complexities. Our expertise encompasses feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting. With over 20 years of experience in Medtech, we specialize in the phases of medical device clinical trials, which include Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) in Latin America.
Our dedicated team ensures that your research studies are conducted effectively and in accordance with local laws, including those established by INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a critical role in medical product oversight as a Level 4 health authority recognized by PAHO/WHO.
The Stages of Medical Device Clinical Trials: From Pilot to Post-Market
The phases of medical device clinical trials typically progress through several critical stages, each playing a vital role in ensuring the safety and effectiveness of innovative technologies. At bioaccess®, we excel in managing these trials, emphasizing innovation and regulatory excellence throughout Latin America.
Early-Feasibility Studies (EFS): These foundational studies are essential for evaluating the viability of new medical devices. They identify potential challenges early in the process and refine study protocols. With over 20 years of Medtech experience, bioaccess® optimizes EFS to ensure they yield valuable insights that inform subsequent phases of medical device clinical trials.
First-In-Human Studies (FIH): Following successful EFS, FIH studies are conducted to assess the device's safety and performance in humans for the first time. Our dedicated team at bioaccess® navigates the complexities of FIH studies, ensuring compliance and excellence throughout the entire process.
Pilot Studies: These small-scale studies are critical for evaluating feasibility and collecting preliminary safety data. They enable researchers to identify potential challenges and refine protocols before launching larger experiments. Recent findings suggest that pilot studies can effectively predict the feasibility of full-scale experiments, with 43% demonstrating equivalent or improved successful screening probabilities compared to their larger counterparts. Additionally, 77% of pilot studies reported enhanced enrollment rates, underscoring their importance in the clinical research landscape. A study titled "Feasibility Parameters in Clinical Trials" assessed key feasibility factors and found that pilot studies are instrumental in estimating the practicality of full-scale experiments, highlighting their predictive capabilities. Bioaccess® leverages its extensive Medtech experience to optimize pilot studies, ensuring they lay a solid foundation for subsequent phases of medical device clinical trials.
After successful preliminary research, the phases of medical device clinical trials involve crucial studies conducted on a larger scale to provide conclusive evidence regarding the apparatus's efficacy and safety. These studies are designed to meet regulatory requirements and often encompass diverse patient populations to guarantee comprehensive data collection. The success rates of pivotal studies for medical devices have shown promising trends, with many achieving their primary objectives, facilitating timely market entry for innovative solutions. Our team at bioaccess® is committed to expertly navigating the complexities of pivotal trials, ensuring compliance and excellence throughout the process.
Post-Market Surveillance: Once a device receives approval, ongoing studies are critical for monitoring its performance in real-world settings. This phase identifies any long-term effects and ensures that the device continues to meet safety standards. The 2023 NIH Policy for Data Management and Sharing underscores the necessity for robust data management plans during this stage, ensuring that findings are accessible and transparent. This policy is particularly relevant as it guides the management of data collected during post-market studies, ensuring compliance and enhancing the reliability of findings. At bioaccess®, we prioritize comprehensive reporting and project management to support our clients in this vital phase.
The importance of pilot studies cannot be overstated, as they establish the groundwork for successful pivotal experiments. Expert opinions emphasize that modifications increasing participant burden may hinder the feasibility of full-scale studies. As Xiangji Ying noted, "Modifications increasing participant burden might reduce full-scale study feasibility," underscoring the need for careful consideration during feasibility evaluations.
As the landscape of medical equipment studies evolves, staying informed about the latest advancements and best practices is essential for optimizing clinical research design and execution. Bioaccess® is here to guide you every step of the way.
Navigating Regulatory Pathways for Medical Device Trials
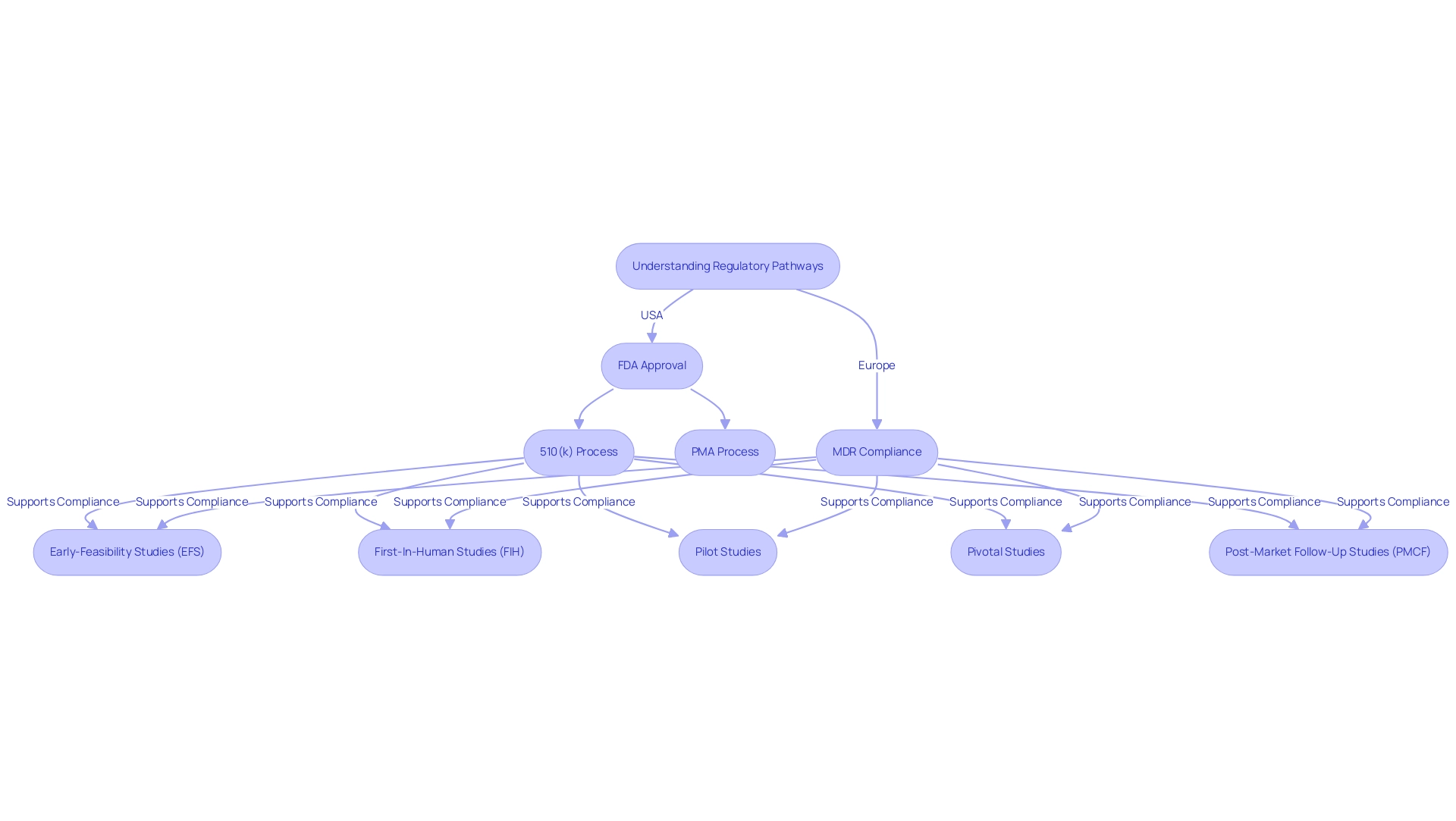
Navigating the regulatory pathways for the phases of medical device clinical trials is essential for successful market entry, as these pathways differ significantly between regions. In the United States, the FDA regulates the approval of products through mechanisms such as the 510(k) premarket notification and the more rigorous Premarket Approval (PMA) process. The 510(k) pathway enables manufacturers to demonstrate that their product is substantially equivalent to an already marketed item, facilitating a quicker route to market.
Conversely, the PMA procedure is designated for high-risk products and necessitates extensive trial data to demonstrate safety and effectiveness.
In Europe, the environment is shaped by the Medical Product Regulation (MDR), which mandates thorough evaluations and post-market monitoring. The MDR underscores the significance of medical evidence, requiring manufacturers to conduct comprehensive studies to support their claims. This regulatory framework has evolved to enhance patient safety and device effectiveness, reflecting a growing demand for transparency and accountability in the approval process.
With over 20 years of experience in the Medtech sector, bioaccess® is well-positioned to assist manufacturers in navigating these complex regulatory pathways. The firm’s distinctive value offering resides in its capacity to connect innovative Medtech firms with unexplored opportunities for conducting research studies in Latin America. Bioaccess® focuses on comprehensive research study management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Additionally, bioaccess® provides feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting. This expertise is especially significant considering the recent increase in the approval of AI/ML-based medical technologies, with 222 products authorized in the USA and 240 in Europe.
Bioaccess® has effectively assisted clients, including Avantec Vascular, in managing these approvals, ensuring adherence to legal standards while promoting the incorporation of advanced technologies in healthcare.
Understanding the phases of medical device clinical trials is vital for manufacturers seeking to simplify their research processes and navigate the intricacies of product authorization. By aligning their strategies with the specific requirements of the FDA and the MDR, companies can enhance their chances of successful market entry and ultimately contribute to advancing medical technology.
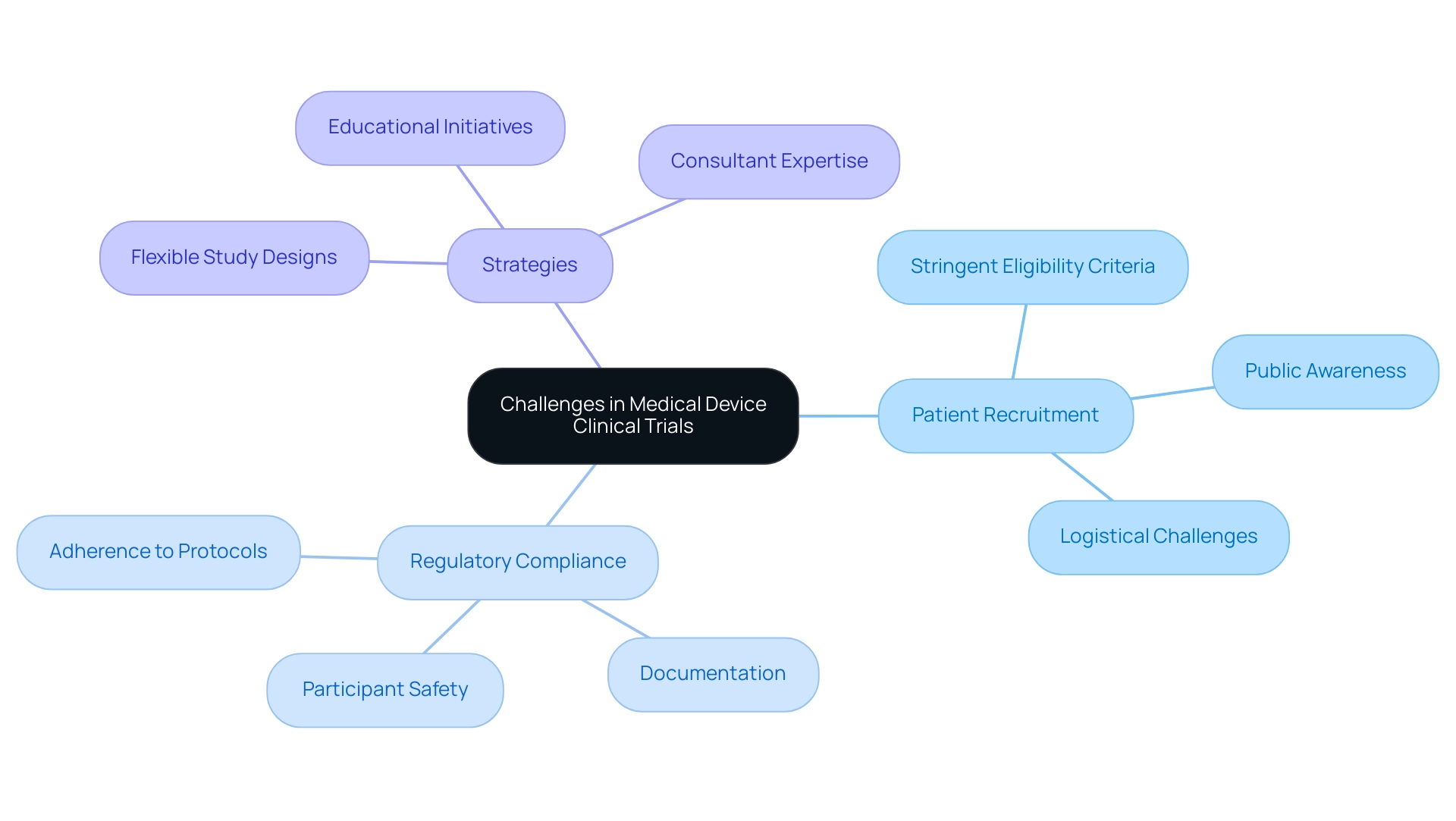
Challenges in Conducting Medical Device Clinical Trials: Recruitment and Compliance
Carrying out medical device studies necessitates navigating a complex landscape filled with challenges, particularly in patient recruitment and regulatory compliance. In 2025, recruitment efforts are often hindered by stringent eligibility criteria, limiting the pool of potential participants. Moreover, a significant barrier is the general lack of public awareness regarding medical studies, exacerbated by logistical challenges such as transportation and scheduling conflicts that can deter participation.
Compliance with regulatory requirements introduces another layer of complexity, demanding strict adherence to established protocols, meticulous documentation, and unwavering commitment to participant safety. Studies reveal that compliance rates in medical device clinical studies can vary significantly, with some experiencing rates as low as 60%. This underscores the critical need for organizations like bioaccess® to implement robust strategies that enhance compliance and ensure the integrity of their studies.
To address these multifaceted challenges, strategic planning and effective communication are essential. Organizations are increasingly prioritizing flexibility in their study designs, enabling adjustments that accommodate participant needs and bolster recruitment efforts. Engaging patients through educational initiatives and outreach programs can also foster greater awareness and interest in research studies.
As noted by MakroCare, "By utilizing the knowledge of consultants, medical device firms can concentrate on what is most important – innovation and patient care – while entrusting the operational challenges of research studies to the specialists."
Furthermore, leveraging the expertise of consultants, such as those at bioaccess®, can provide invaluable support throughout the research process. These professionals assist in creating comprehensive project timelines that include contingency plans for potential delays, ensuring that projects remain on schedule and within budget. A case study on cost management in medical equipment research illustrates how specialized consultants implemented cost-saving measures and monitored study progress, ultimately enabling companies to achieve their objectives efficiently.
In summary, overcoming the challenges of patient recruitment and compliance in medical device research requires a multifaceted approach that integrates innovative strategies, expert guidance, and a commitment to patient involvement. By focusing on these areas, organizations like bioaccess® can significantly enhance their chances of success in the evolving clinical trial landscape, particularly within the dynamic environment of Latin America. Katherine Ruiz's expertise in Regulatory Affairs further highlights the importance of effectively navigating these challenges.
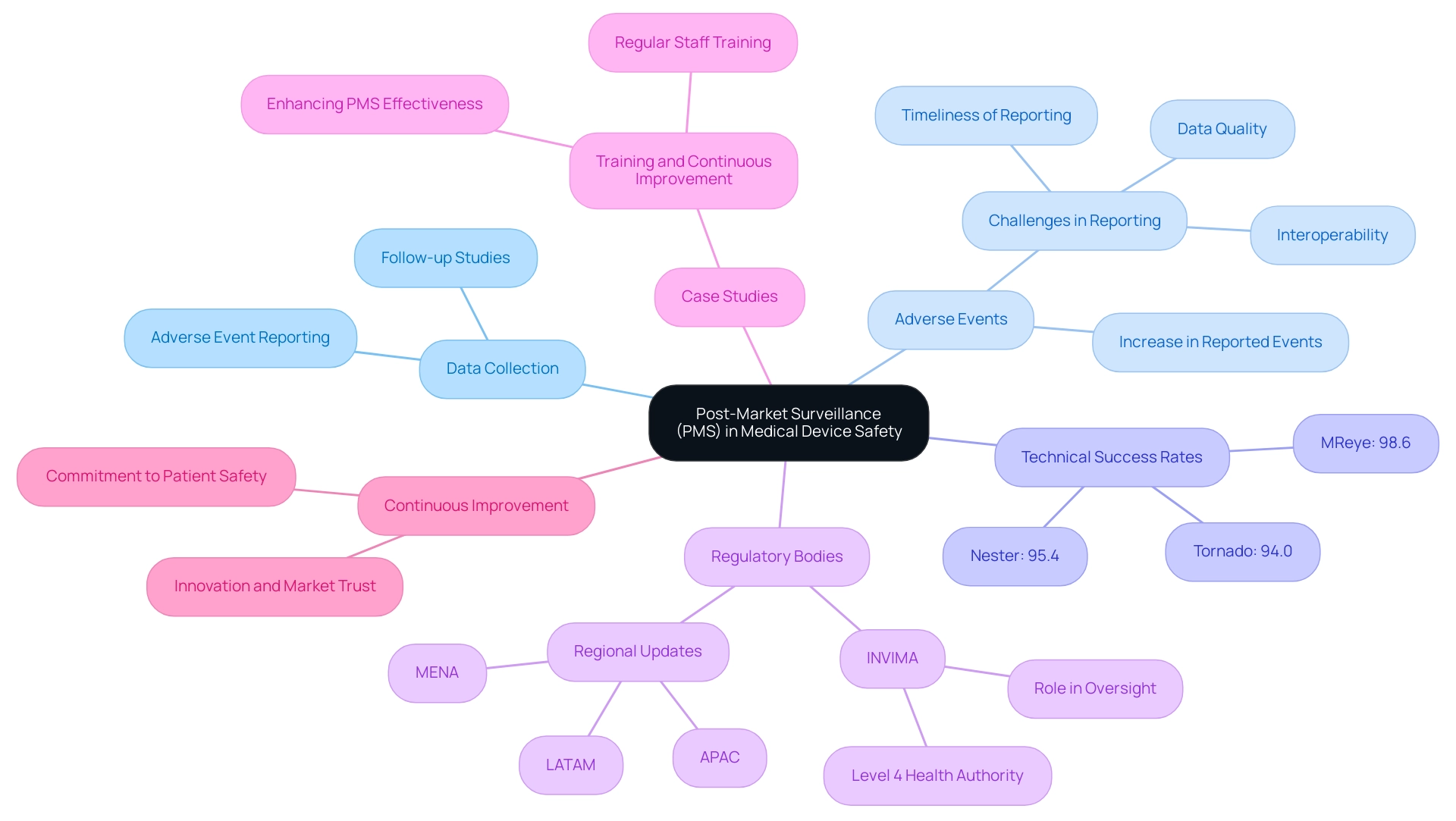
The Role of Post-Market Surveillance in Medical Device Safety
Post-market surveillance (PMS) is a crucial element of the medical product lifecycle, focusing on the continuous evaluation of performance and safety following market approval. This phase encompasses the systematic collection of data on adverse events, the execution of follow-up studies, and the implementation of corrective actions when necessary. Effective PMS is vital for manufacturers, allowing for the early detection of potential safety issues, thereby ensuring that products remain safe and effective for patients.
In 2025, the landscape of post-market surveillance has evolved significantly, with a notable increase in the number of adverse events reported. This uptick underscores the importance of robust data quality, interoperability, and timely reporting mechanisms—critical challenges in the field. Recent statistics indicate that technical success rates for various groups, such as Nester, Tornado, and MReye, were impressively high, at 95.4%, 94.0%, and 98.6%, respectively. These figures highlight the effectiveness of diligent post-market monitoring in enhancing the safety and performance of medical products.
Regulatory bodies, including INVIMA, the Colombia National Food and Drug Surveillance Institute, are increasingly emphasizing the necessity of ongoing reporting and analysis to maintain compliance and safeguard public health. INVIMA plays a crucial role in medical equipment oversight and classification as a Level 4 health authority by PAHO/WHO. Countries across the APAC, LATAM, and MENA regions are updating their regulations to foster innovation while ensuring that safety and quality standards are upheld, particularly in the realms of digital health and telemedicine. This changing compliance landscape presents both opportunities and challenges for manufacturers as they navigate adherence while striving for innovation.
In Latin America, bioaccess® stands out with its accelerated medical technology clinical study services, leveraging over 20 years of expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). This specialized knowledge is crucial for effective PMS, ensuring that manufacturers can respond swiftly to any emerging safety concerns. Katherine Ruiz, a specialist in Compliance Affairs for Medical Devices and In Vitro Diagnostics in Colombia, further enhances bioaccess's capabilities in navigating the complex oversight environment.
A compelling case study on training and continuous improvement illustrates the significance of regular staff training on post-market surveillance procedures. This initiative not only enhances the effectiveness of PMS systems but also contributes to equipment safety, customer satisfaction, and ongoing business improvement. As highlighted by industry experts, "Post-market surveillance is more than a regulatory checkbox. It is an opportunity for manufacturers to underline their commitment to patient safety, propel innovation, and build market trust." This perspective reinforces the critical role of PMS in the medical equipment industry.
In conclusion, the importance of post-market surveillance in ensuring medical device safety cannot be overstated. By implementing effective monitoring strategies and fostering a culture of continuous improvement, manufacturers can significantly enhance patient outcomes and maintain compliance with evolving regulatory standards.
Conclusion
Medical device clinical trials are fundamental in assuring the safety and effectiveness of new technologies prior to their market introduction. The structured phases of these trials, ranging from Early-Feasibility Studies to Post-Market Surveillance, are crucial in navigating regulatory requirements and fostering confidence among healthcare professionals and patients. As the industry progresses, grasping the intricacies of these trials—including classification implications, demographic factors, and the necessity of transparency—becomes increasingly vital for all stakeholders involved.
The obstacles of patient recruitment and regulatory compliance underscore the necessity for strategic planning and expert guidance. By leveraging specialized services, manufacturers can refine their trial processes, ensuring adherence to regulatory standards while effectively engaging potential participants. The focus on post-market surveillance further emphasizes the ongoing commitment to patient safety and device efficacy, reinforcing the imperative for continuous monitoring and enhancement even after market entry.
In conclusion, the pathway of medical devices through clinical trials is complex and demands a unified effort from manufacturers, regulatory bodies, and clinical research organizations. By prioritizing innovation, compliance, and patient engagement, the medical device industry can continue to flourish, ultimately paving the way for the successful introduction of groundbreaking technologies that enhance patient outcomes and advance healthcare as a whole.
Frequently Asked Questions
What are medical equipment research studies?
Medical equipment research studies are organized examinations aimed at assessing the safety and efficacy of novel medical instruments, ensuring that products comply with standards before their market introduction.
Why are clinical trials important in the medical device sector?
Clinical trials are essential for regulatory approval and instilling confidence among healthcare providers and patients regarding the safety and effectiveness of medical devices.
What does the KAMRA corneal implant study reveal about patient demographics?
The KAMRA study indicated that patients aged 45-49 years had a significantly higher likelihood (88%) of achieving improved near vision compared to those aged 55-60 years (78%) after one year of treatment, highlighting the importance of demographic factors in medical evaluations.
What is the significance of subgroup analyses in medical studies?
Subgroup analyses are important as they provide insights into how different demographics respond to treatments; however, they are often underreported in public reviews and official device labeling.
What concerns exist regarding mandatory disclosure requirements for medical device manufacturers?
There are concerns that mandatory disclosure requirements may lead manufacturers to conduct investigations offshore to protect confidentiality, which could affect the accessibility of essential information for oversight decisions and medical practice.
What services does bioaccess® provide in the medical apparatus sector?
Bioaccess® specializes in extensive research study management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, tailored to the medical apparatus sector in Latin America.
How are medical instruments classified?
Medical instruments are categorized into three primary classes: Class I (low risk), Class II (moderate risk), and Class III (high risk), each with specific compliance demands that influence research procedures for gaining approval.
What is the difference in oversight between Class I and Class III medical devices?
Class I devices generally undergo limited oversight and are often exempt from premarket notification, while Class III devices face rigorous evaluations to thoroughly assess their safety and effectiveness prior to market entry.
How does product classification impact research strategies for manufacturers?
Product classification affects trial design, patient recruitment, and overall study timelines, with Class II devices requiring a 510(k) submission and Class III devices needing comprehensive clinical data through Investigational Device Exemptions (IDEs).
What trends are emerging in the oversight landscape for medical devices?
There is an increasing emphasis on risk management and post-market surveillance, particularly for advanced therapies like gene editing and mRNA technologies, necessitating adaptive governance frameworks.
What role does INVIMA play in medical product oversight in Colombia?
INVIMA, as Colombia's Level 4 health authority, ensures compliance and oversight throughout the testing process for medical products, playing a critical role in the governance landscape.




