Overview
The article emphasizes the optimization of medical device trial strategies in Chile, capitalizing on the country's geographic, demographic, and regulatory strengths. It asserts that Chile's diverse population, efficient regulatory processes, and commitment to healthcare innovation foster a favorable environment for conducting clinical trials. This environment not only enhances participant recruitment but also significantly improves research outcomes, making Chile a prime location for clinical research initiatives.
Introduction
Chile stands at the forefront of clinical research, showcasing a unique blend of geographic diversity, demographic richness, and a steadfast commitment to innovation. This combination positions it as an ideal hub for clinical trials.
With urban centers that are primed for participant recruitment and a regulatory framework that streamlines approvals, the nation presents an attractive landscape for sponsors eager to advance medical technologies.
As investment in the clinical trial sector surges, the benefits of conducting research in Chile become increasingly apparent, ranging from cost savings to access to skilled professionals and cutting-edge facilities.
This article delves into how leveraging these elements can enhance the efficiency and effectiveness of clinical trials, ultimately benefiting both researchers and patients alike.
Leverage Chile's Geographic and Demographic Advantages for Clinical Trials
Leverage Chile's Geographic and Demographic Advantages for Clinical Trials
Chile's geographic diversity and rich demographic characteristics position it as an optimal location for conducting clinical trials. The nation features a population that is not only ethnically diverse but also exhibits a wide range of health conditions, particularly concentrated in urban areas. This urban density facilitates easier access to potential participants, significantly enhancing recruitment efficiency. Furthermore, the country's advantageous time zone aligns well with North American and European markets, facilitating smooth communication and collaboration among research teams.
The yearly funding in the research sector in Latin America’s Andean Region has surged from $3-4 million to over $50 million annually, underscoring the rising significance of this area for medical studies. For instance, a recent medical study for a cardiovascular device effectively gathered participants from both urban and rural environments, demonstrating the benefits of leveraging the demographic strengths of the region. As Julio G. Martinez-Clark, CEO of bioaccess, noted, 'With its swift and thorough approval processes, Chile offers an attractive landscape for medical device trial strategies in Chile, enabling startups to innovate.'
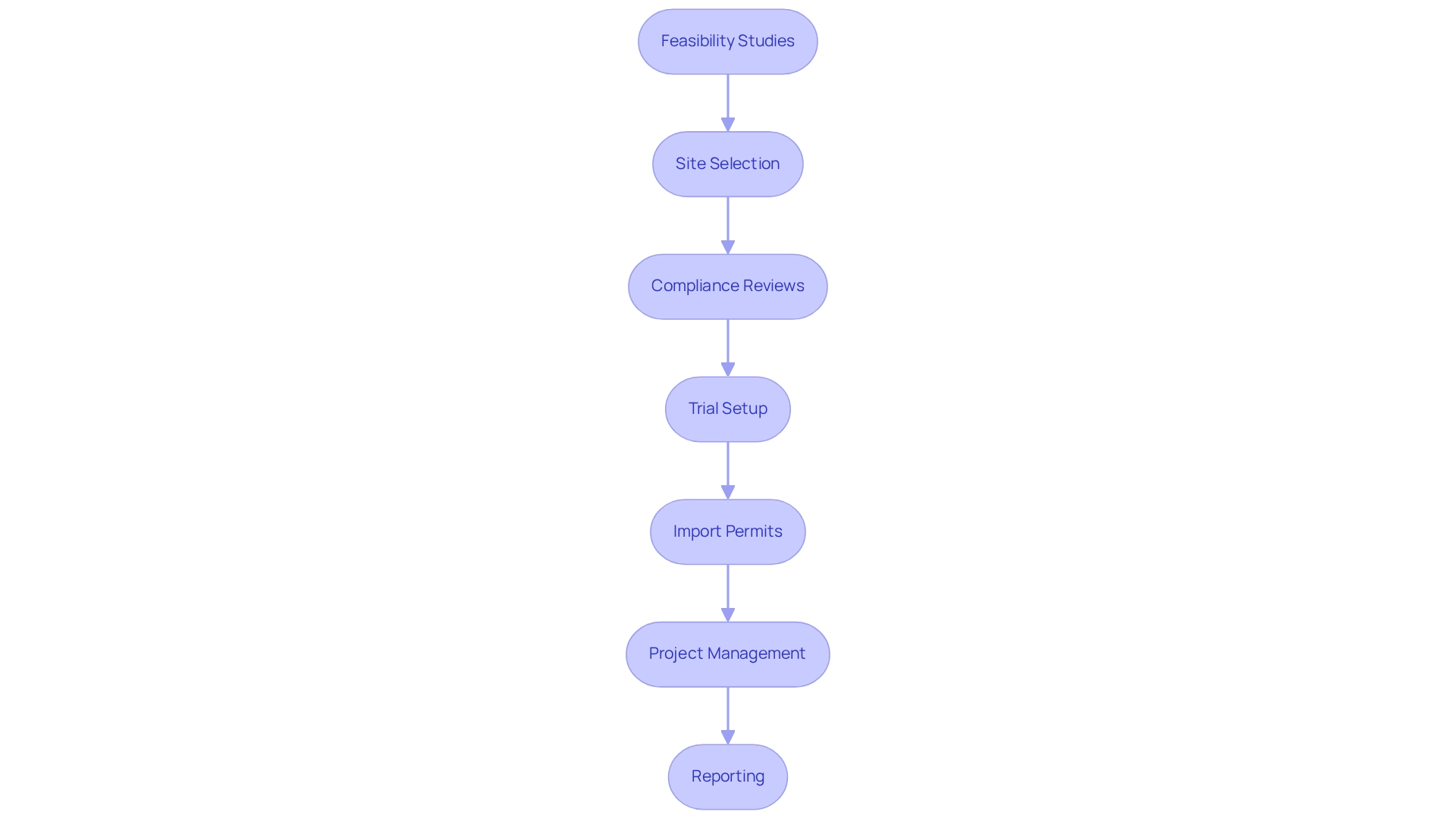
To fully capitalize on these advantages, research sponsors should conduct thorough demographic analyses to identify target groups that align with their study goals. Engaging local healthcare providers can enhance outreach efforts and increase participant enrollment rates, ultimately leading to more robust and representative research outcomes. Furthermore, bioaccess® provides comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services ensure that trials are conducted efficiently and effectively. It is also vital to consider cultural differences and socio-economic factors to improve patient safety and the quality of medical studies in Latin America. As Wenting Wang, Global Head of Biomarker Statistics, emphasized, involving varied populations in research studies is essential for producing insights that genuinely represent our global society. Moreover, the influence of Medtech research on local economies, such as job generation and healthcare enhancement, cannot be overlooked, emphasizing the broader advantages of conducting studies in this area.
Navigate the Regulatory Framework Supporting Clinical Trials in Chile
Navigate the Regulatory Framework Supporting Clinical Trials in Chile
Chile's regulatory environment for clinical trials is primarily overseen by the Instituto de Salud Pública (ISP), which enforces stringent ethical standards to ensure participant safety and data integrity. The typical approval duration for medical studies in Chile is around 3-4 months, placing the nation advantageously relative to other areas. Remarkably, 89% of sponsors are presently utilizing decentralized technology to aid with at least one of their experiments, indicating a significant trend in the research landscape.
To effectively navigate this framework, sponsors should consider the following steps:
- Develop Comprehensive Study Protocols: Ensure that study protocols comply with local regulations and engage accredited Ethics Committees early in the process.
- Stay Informed on Regulatory Updates: Keep abreast of recent regulatory updates, including the ISP's alignment with international standards, to gain significant advantages.
- Proactive Communication: For instance, a recent clinical trial involving an innovative orthopedic device exemplified this approach; proactive communication with regulatory authorities led to expedited approval and successful patient recruitment.
Furthermore, the strong regulatory system in the nation encourages innovation in healthcare technology, particularly through medical device trial strategies in Chile, positioning it as a competitive participant in the international device field. As emphasized by the bioaccess® content team, "The dedication to strict standards and ethical practices establishes Chile as a competitive participant in the global healthcare device arena." By thoroughly grasping and utilizing the regulatory environment, sponsors can improve operational efficiency and ensure adherence throughout the study lifecycle, ultimately aiding the timely progression of medical device trial strategies in Chile to bring medical devices to the market. Furthermore, tackling linguistic, cultural, and socio-economic obstacles is essential for enhancing the quality of medical studies and healthcare in the area.
Utilize Access to Skilled Medical Professionals and Research Facilities
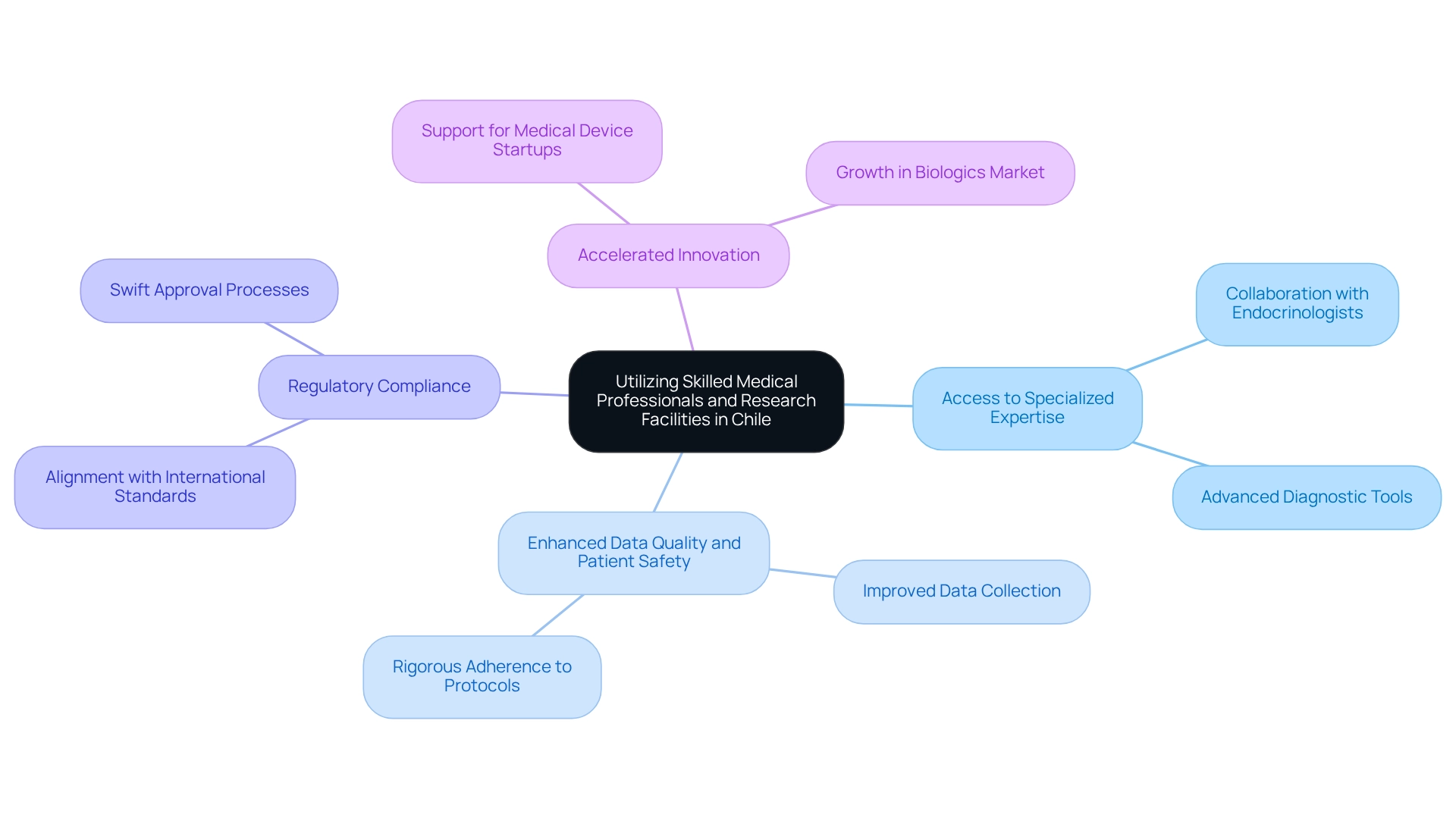
Chile boasts a robust network of proficient healthcare experts, including doctors, nurses, and researchers, who possess significant knowledge of medical device trial strategies. This expertise is further supported by advanced research facilities equipped with innovative technology, establishing the country as an attractive location for medical device trial strategies in 2025. Collaborating with local healthcare organizations not only enhances the quality of data collected but also significantly improves patient safety during the testing process, which is critical for effective medical device trial strategies in Chile.
A notable example is a recent study on a diabetes management device conducted at a leading hospital in Santiago. This collaboration provided access to specialized endocrinologists and advanced diagnostic tools, enabling efficient patient monitoring and ensuring rigorous adherence to medical protocols. Such partnerships are essential for executing medical device trial strategies in Chile; they allow sponsors to leverage local expertise, enhance study outcomes, and bolster the credibility of their research.
Moreover, the presence of qualified healthcare professionals in the region underpins a healthcare system that meets global standards, which is vital for the successful implementation of medical device trial strategies in Chile, guaranteeing that studies are conducted with the highest quality. With the biologics market projected to expand at a compound annual growth rate of 15% until 2027, the urgency of establishing local healthcare partnerships becomes increasingly paramount. Engaging with local specialists promotes innovation and accelerates the development of medical device trial strategies in Chile, ultimately benefiting both patients and the healthcare system.
Key advantages of local partnerships in the region include:
- Access to specialized medical expertise and advanced diagnostic tools, crucial for comprehensive research management services.
- Enhanced quality of data collection and improved patient safety, essential components of medical device trial strategies in Chile, addressing common challenges faced by medical device startups.
- Alignment with international regulatory standards, emphasized by the region's cutting-edge regulatory framework for research, which supports medical device trial strategies in Chile, facilitating compliance reviews and setup.
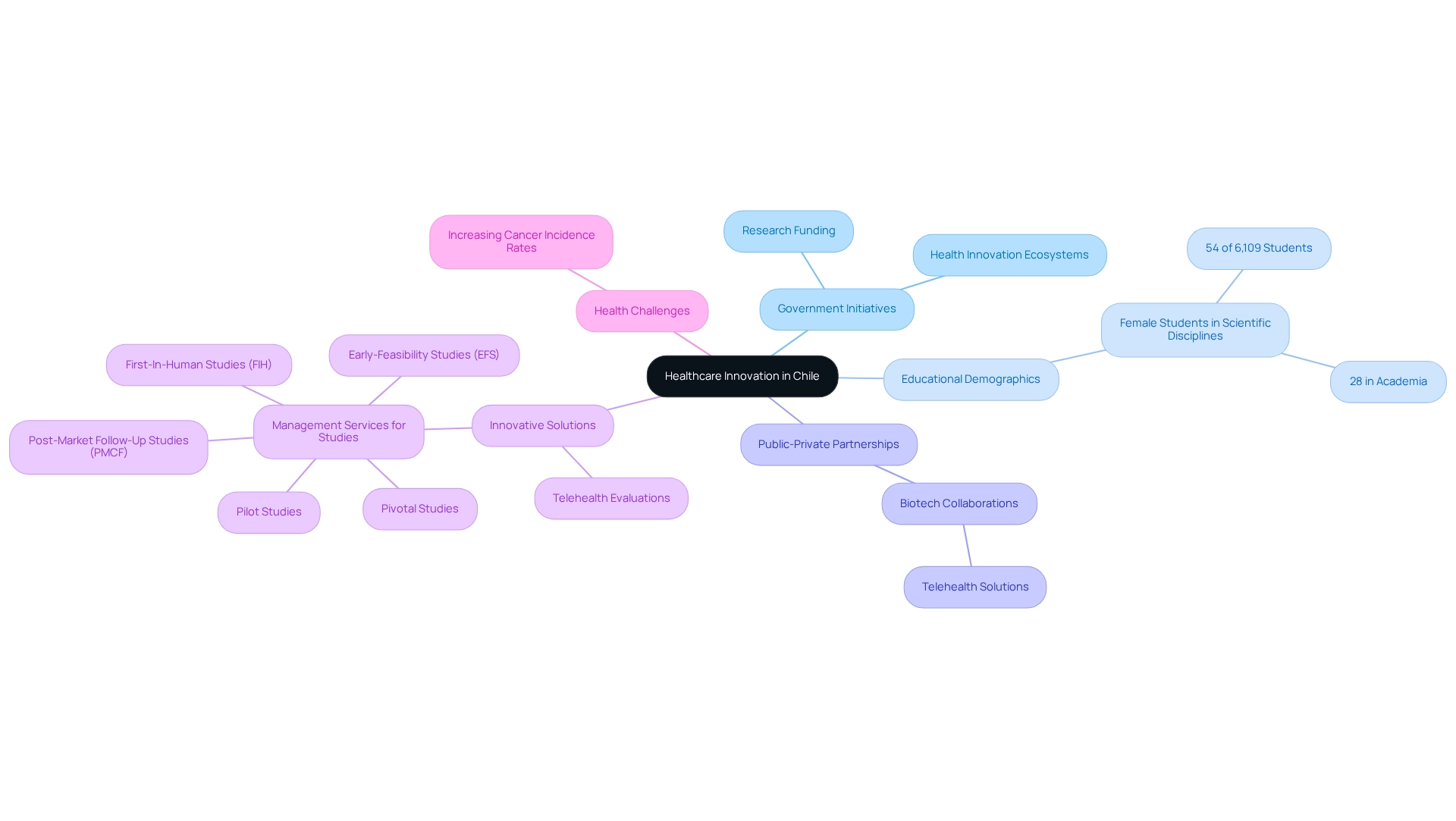
- Accelerated innovation and development of medical devices in a growing market, supported by bioaccess®'s expertise in managing medical device trial strategies in Chile, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
To learn more about how bioaccess® can help optimize your research in the region, contact us today.
Maximize Cost-Effectiveness in Conducting Trials in Chile
Maximize Cost-Effectiveness in Conducting Trials in Chile
Conducting clinical trials in Chile presents substantial cost advantages compared to North America and Europe, with operational expenses potentially reduced by up to 30%. This financial benefit arises from lower labor costs, decreased overhead for facilities, and streamlined patient recruitment strategies. For instance, in 98.4% of simulations, lenalidomide plus dexamethasone was determined to be cost-effective compared to bortezomib plus dexamethasone, underscoring the potential for considerable savings in clinical study costs. By leveraging these advantages, sponsors can allocate resources more effectively across their project portfolios.
A medtech company conducting a crucial study for a cardiac device discovered that employing medical device trial strategies in Chile not only reduced costs but also accelerated patient enrollment. This strategic approach enabled the company to enhance its return on investment while ensuring the successful execution of its research studies. Julio G. Martinez-Clark, CEO of bioaccess, noted that 'Colombia and Paraguay have emerged as the most prominent nations in Latin America for EFS and FIH studies,' highlighting an increasing recognition of the region's potential for medical research. Moreover, regional healthcare programs in the southern part of the country are expanding, fostering a supportive environment for drug distribution systems and research initiatives.
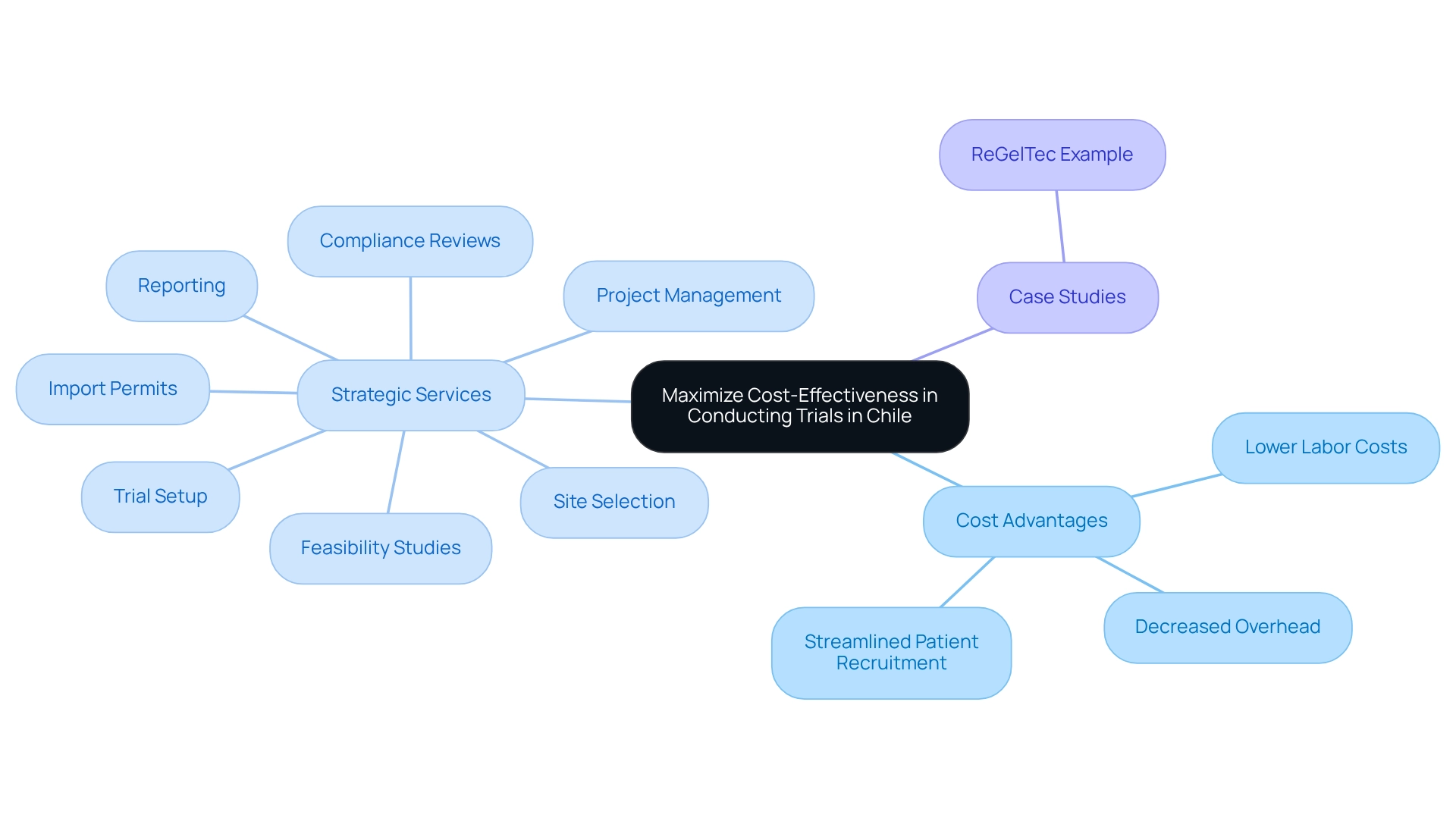
To maximize the benefits of conducting trials in Chile, bioaccess provides a comprehensive range of clinical trial management services, including:
- Feasibility Studies
- Site Selection
- Compliance Reviews
- Trial Setup
- Import Permits
- Project Management
- Reporting
These services ensure that sponsors can navigate the complexities of clinical trials effectively, further enhancing their success in this promising market. Additionally, the ReGelTec case study exemplifies how bioaccess's expertise facilitated the successful enrollment and treatment of patients in their Early Feasibility Study for HYDRAFIL™, showcasing the practical application of these services in real-world scenarios.
These examples illustrate the opportunity for medtech firms to achieve significant financial gains by implementing medical device trial strategies in Chile, establishing it as a compelling choice for research in the field.
Capitalize on Chile's Commitment to Innovation in Healthcare
The healthcare system in the country is increasingly prioritizing innovation, driven by government initiatives that promote research and development in healthcare technologies. This commitment is evident in the establishment of health innovation ecosystems and partnerships between public and private sectors, creating a supportive environment for research in healthcare. For example, 54% of the 6,109 students in scientific disciplines at the University of Chile are female, underscoring the diverse talent pool available for medical technology research. Sponsors can leverage this environment by aligning their research studies with local innovation agendas, potentially unlocking opportunities for funding and additional support.
A notable example is the collaboration between a biotech company and a Chilean university that resulted in an innovative telehealth solution, which has been effectively evaluated in health assessments. Such partnerships not only enhance visibility for sponsors but also foster connections that can streamline testing procedures and facilitate the adoption of new technologies in the market. With bioaccess®'s comprehensive management services for studies—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF)—sponsors can navigate the complexities of research with greater efficiency.
As Warren Bennis aptly stated, "Innovation—any new idea—by definition will not be accepted at first. It takes repeated attempts, endless demonstrations, monotonous rehearsals before innovation can be accepted and internalized by an organization. This requires courageous patience." As Chile advances with its healthcare innovation projects, aligning with these efforts will be crucial for the success of medical device trial strategies in Chile and the wider Medtech landscape. Furthermore, addressing specific health challenges, such as the increasing cancer incidence rates, through innovative solutions will be vital in shaping the future of healthcare in the country. The impact of Medtech clinical studies on local economies, including job creation and healthcare enhancement, highlights the significance of these initiatives.
Conclusion
Chile's unique blend of geographic diversity, demographic richness, and a robust regulatory framework establishes it as an ideal location for conducting clinical trials. The country's urban centers significantly enhance participant recruitment, while its favorable time zone facilitates seamless collaboration with international research teams. As investment in the clinical trial sector continues to grow, the advantages of conducting research in Chile become increasingly evident, encompassing cost savings, access to skilled professionals, and advanced facilities.
The commitment to innovation within Chile's healthcare system further amplifies its appeal for clinical trials. Government initiatives supporting research and development foster an environment ripe for collaboration between public and private sectors, leading to groundbreaking advancements in medical technology. Engaging local healthcare providers and leveraging the expertise of skilled professionals can significantly enhance trial quality and patient safety, ultimately resulting in more reliable outcomes.
Moreover, the cost-effectiveness of conducting trials in Chile positions it as a strategic option for sponsors aiming to maximize their resources. With operational expenses potentially reduced by up to 30% compared to North America and Europe, clinical trial sponsors can allocate their budgets more efficiently, thereby enhancing their return on investment.
In conclusion, as Chile continues to strengthen its position as a leader in clinical research, the benefits of conducting trials in this region are clear. By capitalizing on its diverse population, streamlined regulatory processes, skilled workforce, and commitment to innovation, sponsors can enhance the efficiency and effectiveness of their clinical trials. This not only serves the interests of researchers but also significantly benefits patients, paving the way for advancements in healthcare that can resonate globally.
Frequently Asked Questions
What advantages does Chile offer for conducting clinical trials?
Chile's geographic diversity and rich demographic characteristics make it an optimal location for clinical trials. The population is ethnically diverse and has a wide range of health conditions, especially in urban areas, enhancing recruitment efficiency. Additionally, Chile's time zone aligns well with North American and European markets, facilitating communication and collaboration.
How has funding for research in Chile changed in recent years?
Funding in the research sector in Latin America’s Andean Region has surged from $3-4 million to over $50 million annually, highlighting the growing significance of the area for medical studies.
What role do local healthcare providers play in clinical trials in Chile?
Engaging local healthcare providers can enhance outreach efforts and increase participant enrollment rates, leading to more robust and representative research outcomes.
What services does bioaccess® provide for clinical trial management?
bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Why is it important to consider cultural differences and socio-economic factors in clinical trials?
Considering cultural differences and socio-economic factors is vital for improving patient safety and the quality of medical studies in Latin America, ensuring that research insights genuinely represent diverse populations.
What is the regulatory framework for clinical trials in Chile?
The regulatory environment for clinical trials in Chile is overseen by the Instituto de Salud Pública (ISP), which enforces ethical standards to ensure participant safety and data integrity.
How long does it typically take to get approval for medical studies in Chile?
The typical approval duration for medical studies in Chile is around 3-4 months, which is relatively quick compared to other regions.
What steps should sponsors take to navigate the regulatory framework in Chile?
Sponsors should develop comprehensive study protocols that comply with local regulations, stay informed on regulatory updates, and maintain proactive communication with regulatory authorities.
How does the regulatory system in Chile encourage innovation in healthcare technology?
The strong regulatory system supports innovation, particularly through medical device trial strategies, positioning Chile as a competitive player in the international healthcare device market.
What challenges must be addressed to enhance the quality of medical studies in Chile?
Tackling linguistic, cultural, and socio-economic obstacles is essential for improving the quality of medical studies and healthcare in the region.




