Overview
This article emphasizes the crucial steps necessary for designing effective post-market studies in Peru, highlighting the significance of real-world evaluations of medical devices following their market release. It articulates essential considerations, including:
- The definition of clear objectives
- The selection of appropriate study populations
- Strict adherence to regulatory frameworks
These elements not only ensure compliance but also enhance the efficacy of medical technology in improving patient outcomes. Understanding the Medtech landscape is vital, as it presents unique challenges that require strategic collaboration among stakeholders. The role of bioaccess in addressing these challenges cannot be overstated, as it fosters a cooperative environment essential for advancing clinical research. In conclusion, the importance of collaboration in post-market studies is paramount, and the next steps involve engaging with relevant parties to drive innovation and improve patient care.
Introduction
In the dynamic landscape of healthcare, the significance of post-market studies is paramount, especially in Peru, where these evaluations are essential for ensuring the safety and efficacy of medical devices after they enter the market. Companies are compelled to navigate the complexities of regulatory compliance and real-world performance, making a thorough understanding of post-market research indispensable.
These studies not only identify potential adverse events but also cultivate trust among healthcare providers and patients, thereby paving the way for advancements in medical technology.
With a favorable regulatory environment and local expertise, organizations are positioned to effectively harness the power of real-world evidence, ultimately enhancing patient outcomes and driving innovation within the healthcare sector.
Understand the Importance of Post-Market Studies in Peru
Post-market evaluations are crucial for assessing the effectiveness of medical instruments after their market launch. In Peru, the post-market study design for Peru plays a vital role in gathering real-world evidence about the safety and effectiveness of devices, often diverging from the outcomes observed in clinical trials. They are indispensable for identifying adverse events or complications that may arise during everyday use. By executing comprehensive evaluations post-market release, companies not only ensure compliance with local regulations but also build trust among healthcare providers and patients. Furthermore, these investigations yield valuable insights that can drive product enhancements and inform regulatory decisions, ultimately improving patient outcomes and advancing medical technology throughout the region.
bioaccess® specializes in managing a variety of research initiatives, including:
- Early-Feasibility Assessments (EFS)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Trials (PMCF)
With the expertise required to navigate the complexities of these processes in Latin America, bioaccess® is ideally positioned to support companies in their after-sales efforts. The favorable regulatory landscape in Peru, characterized by efficient approval processes and government support for innovation, emphasizes the significance of the post-market study design for Peru. This environment not only encourages investment in the healthcare sector but also facilitates the introduction of new medical technologies into the market. Additionally, according to Global Health Intelligence, leveraging real-world evidence from after-market analyses will be critical for firms aiming to navigate the complexities of the healthcare environment effectively. As the medical equipment market continues to evolve, insights from the post-market study design for Peru will be essential for ensuring the ongoing safety and efficacy of medical tools.

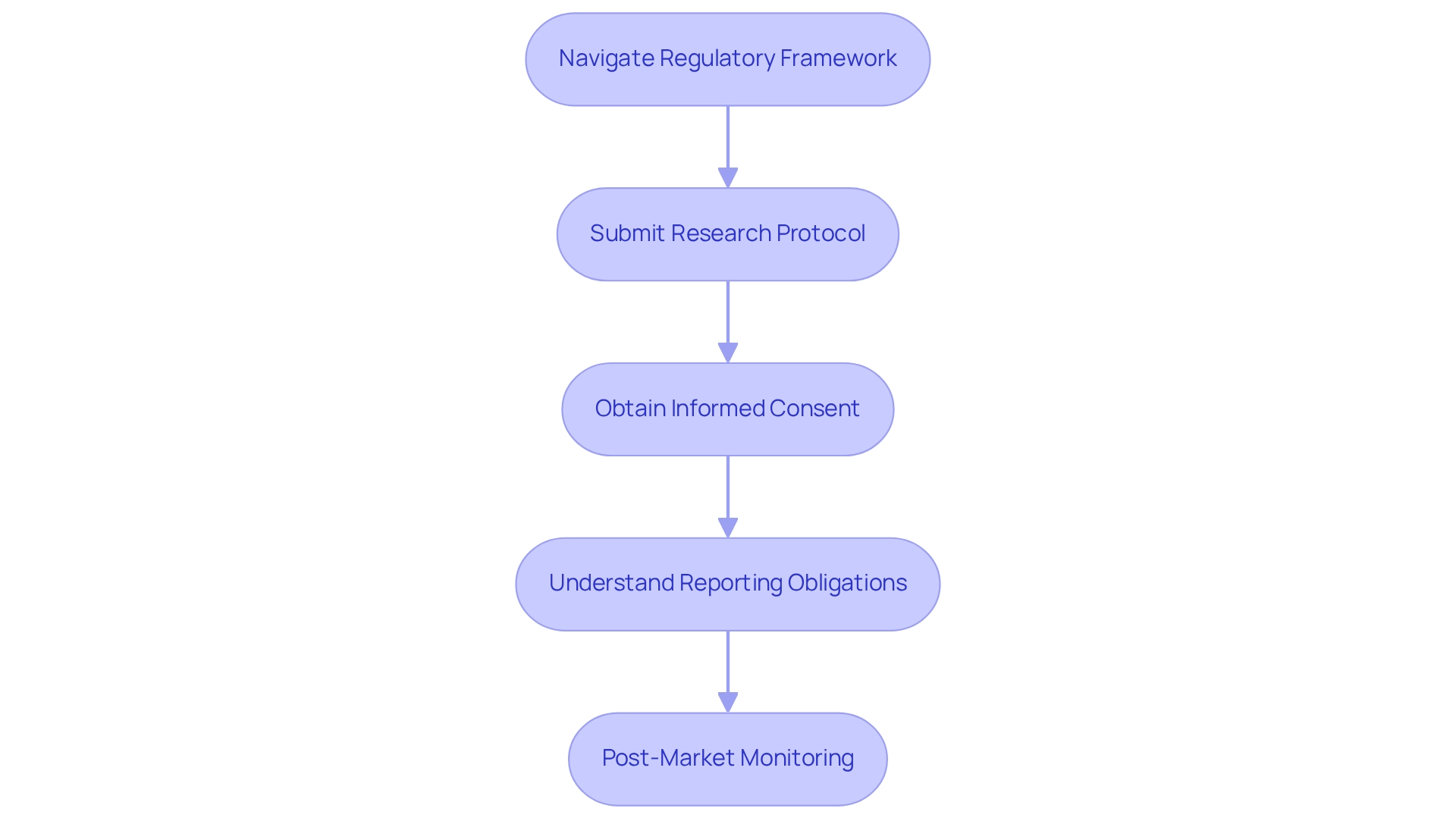
Navigate Peru's Regulatory Framework for Medical Devices
A thorough understanding of the regulatory framework established by the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID) is essential for the post-market study design for Peru. This framework ensures the safety, efficacy, and quality of medical devices, aligning with international standards. Companies must navigate the approval process by submitting a detailed research protocol that outlines the project's objectives, methodology, and anticipated outcomes.
Adhering to ethical standards is essential, necessitating informed consent from participants to uphold the integrity of the research, especially in the context of the post-market study design for Peru, as the regulatory environment remains dynamic with DIGEMID emphasizing the importance of post-market monitoring. Companies are expected to familiarize themselves with specific requirements, including reporting obligations and timelines, which are critical for a seamless approval process. Understanding these components not only aids in compliance but also minimizes the risk of regulatory challenges.
bioaccess® offers a comprehensive suite of clinical trial management services tailored to navigate these complexities. This includes:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial organization
- Import permits
- Project oversight
- Reporting
A significant case analysis illustrates the regulatory structure for medical devices in Peru, outlining the essential steps from initial concept to post-market monitoring. This guide serves as a vital resource for companies aiming to effectively navigate DIGEMID regulations, ensuring their post-market study design for Peru meets all compliance standards. By leveraging local expertise and grasping the nuances of the regulatory landscape, companies can significantly enhance their prospects for successful market entry and ongoing compliance.
Plan Your Post-Market Study Design: Key Considerations
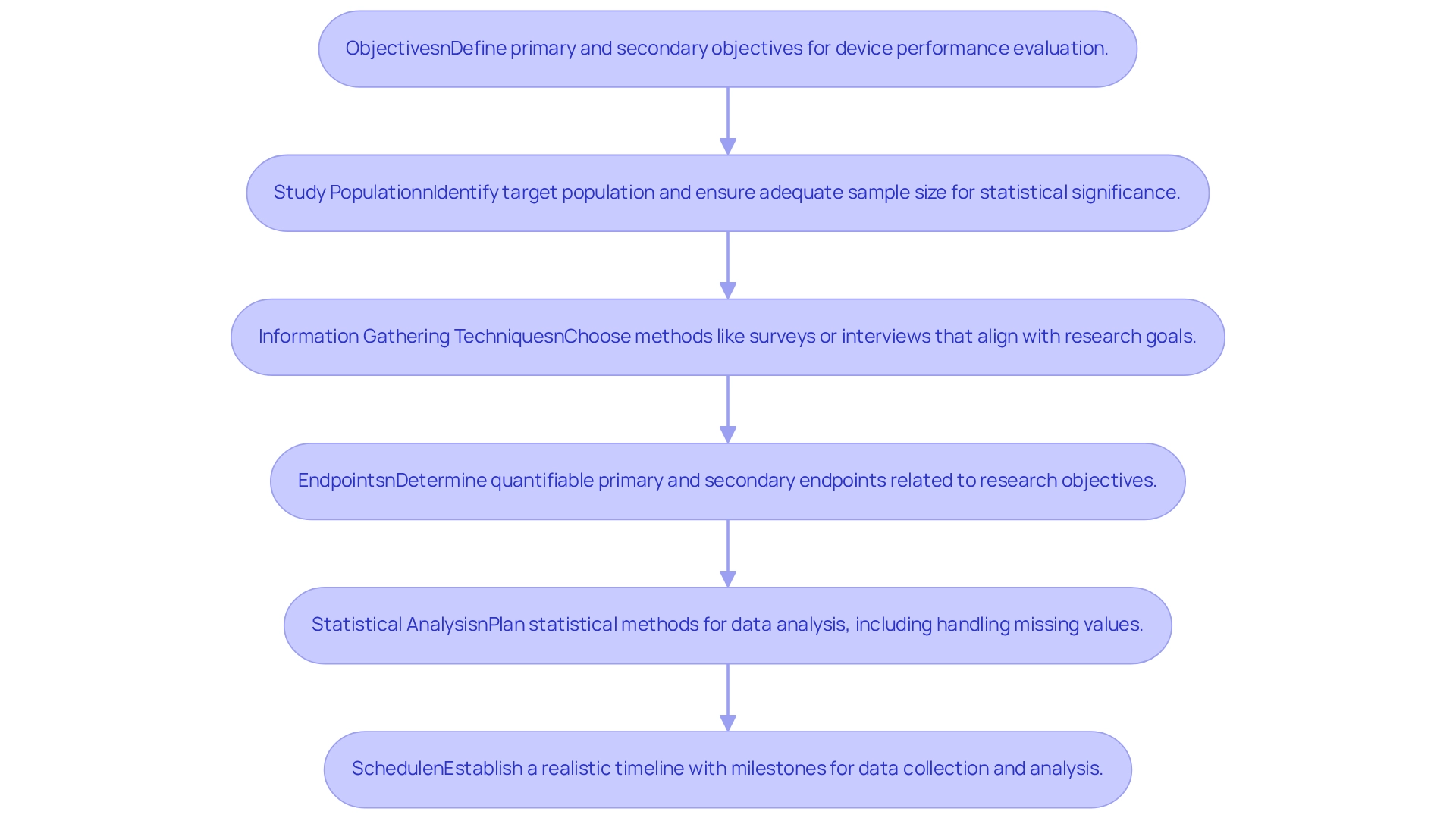
When planning your post-market study design, several key elements must be considered to ensure a comprehensive evaluation and compliance:
-
Objectives: Clearly define both primary and secondary objectives. What specific questions do you aim to answer regarding the device's performance? Setting clear goals is crucial for directing the research and ensuring relevant data gathering.
-
Study Population: Identify the target population for your study. In Peru, the post-market study design for Peru must ensure that the sample size is adequate to provide statistically significant results, as neglecting this can lead to unreliable conclusions and potential regulatory repercussions. Manufacturers neglecting post-market surveillance regulations may face severe repercussions, including fines and product recalls.
-
Information Gathering Techniques: Choose suitable methods for information collection, such as surveys, interviews, or electronic health records. These methods should align with your research goals to ensure that the data collected is pertinent and actionable.
-
Endpoints: Determine the primary and secondary endpoints that will evaluate the device's performance. These endpoints ought to be quantifiable and directly connected to the research objectives, enabling a clear evaluation of outcomes.
-
Statistical Analysis: Plan the statistical methods for information analysis, including strategies for managing missing values and addressing potential confounding factors. This planning is vital for ensuring the integrity of your findings.
-
Schedule: Establish a realistic schedule for the research, incorporating key milestones and deadlines for data collection and analysis. A well-structured timeline is essential for the post-market study design for Peru, as it helps maintain project momentum and ensures timely insights, enabling you to develop a strong research framework that provides valuable insights into the performance of your medical instrument.
As bioaccess® oversees clinical follow-up research after market introduction and provides services like feasibility assessments, investigator selection, regulatory compliance, and project management, they can assist manufacturers in navigating the complexities of trials after market launch. Utilizing various research designs, as emphasized in the case analysis 'Diverse Research Designs in Post-Market Trials,' improves the capacity to oversee device safety and effectiveness, ultimately guiding regulatory choices and enhancing patient outcomes.
Execute the Study: Implementing Your Post-Market Strategy
To successfully carry out your post-market research, follow these essential steps:
- Recruit Participants: Initiate participant recruitment in alignment with your research protocol, ensuring compliance with the defined inclusion and exclusion criteria. Notably, research indicates that a significant number of potential participants may not respond due to a general lack of interest, underscoring the necessity for effective engagement strategies. Furthermore, it is crucial to adopt flexible recruitment strategies, as successful campaigns often require adaptability to various regional contexts. Leveraging bioaccess®'s expertise in managing Early-Feasibility Studies and Post-Market Clinical Follow-Up Studies can enhance your recruitment efforts, particularly in Latin America, where local insights can significantly improve participant engagement.
- Training and Orientation: Conduct comprehensive training for all personnel involved in the study. This training should encompass their specific roles, information collection methods, and ethical considerations, ensuring that everyone is well-prepared to contribute effectively. Consider implementing training programs that focus on practical applications and real-world scenarios to enhance the learning experience. Bioaccess® offers tailored training solutions that can assist your team in navigating the complexities of clinical trials in diverse environments.
- Information Gathering: Execute your intended information gathering techniques meticulously. Close supervision is vital to guarantee adherence to the study protocol and to address any arising issues swiftly, which is crucial for preserving information integrity. Utilizing bioaccess®'s advanced project management tools can streamline this process and improve information accuracy.
- Quality Control: Establish robust quality assurance measures to safeguard the integrity of the gathered information. Frequent audits and inspections can assist in confirming information accuracy and detecting any inconsistencies early in the process. Bioaccess® emphasizes quality assurance during the clinical trial process, ensuring that your research meets the highest standards.
- Information Evaluation: After completing the information gathering, proceed with the statistical examination as outlined in your research framework. It is essential to interpret the results within the context of your research objectives, drawing meaningful conclusions that can inform future practices. Collaborating with bioaccess® can provide access to experienced analysts who can help interpret your data effectively.
- Reporting: Compile a comprehensive report detailing the research findings, including any adverse events or complications encountered. This report should be shared with relevant stakeholders, including regulatory bodies and healthcare providers, to ensure transparency and facilitate informed decision-making. Highlighting the significance of transparency aligns with the necessity for patient participation in the research, as underscored in recent case analyses. Bioaccess®'s commitment to openness and collaboration with regional health agencies, such as the assistance from Colombia's Minister of Health, can bolster the reliability of your results in the context of the post-market study design for Peru.
By adhering to these steps, you can efficiently execute your post-market research approach, providing significant insights for the continuous assessment of your medical device. Involving patients early in the design phase can further enhance recruitment efforts and align the project with patient needs, ultimately improving response rates and outcomes. The case study illustrates how minimal patient involvement may have contributed to lower response rates, reinforcing the importance of their engagement.

Conclusion
Post-market studies are pivotal in the healthcare landscape of Peru, particularly for assessing the real-world performance of medical devices following their market introduction. These studies not only ensure compliance with regulatory frameworks but also enhance the safety and efficacy of medical devices through ongoing monitoring and evaluation. By identifying potential adverse events and fostering trust among healthcare providers and patients, post-market studies are essential for driving innovation and improving patient outcomes.
Navigating the regulatory environment established by DIGEMID is crucial for companies aiming to conduct effective post-market studies. A thorough understanding of the specific requirements, including ethical guidelines and reporting obligations, is vital for a seamless approval process. With local expertise and a favorable regulatory climate, organizations can leverage real-world evidence to make informed decisions and enhance their products.
When designing and executing post-market studies, meticulous planning and execution are paramount. Defining clear objectives, selecting appropriate study populations, and employing robust data collection methods are essential steps in obtaining reliable insights. Moreover, engaging patients throughout the study process not only enhances recruitment efforts but also aligns the research with patient needs, ultimately leading to superior study outcomes.
In conclusion, the significance of post-market studies in Peru cannot be overstated. As the medical device market continues to evolve, these evaluations will be instrumental in ensuring ongoing safety, efficacy, and innovation in healthcare. Embracing the complexities of post-market research empowers organizations to contribute meaningfully to the advancement of medical technology and the enhancement of patient care.
Frequently Asked Questions
Why are post-market evaluations important for medical instruments?
Post-market evaluations are crucial for assessing the effectiveness of medical instruments after their market launch, as they gather real-world evidence about safety and effectiveness, identify adverse events, and ensure compliance with local regulations.
How does the post-market study design in Peru differ from clinical trials?
The post-market study design in Peru often diverges from the outcomes observed in clinical trials, focusing on real-world evidence and the actual performance of devices during everyday use.
What benefits do companies gain from conducting comprehensive post-market evaluations?
Companies benefit by ensuring compliance with local regulations, building trust among healthcare providers and patients, gaining valuable insights for product enhancements, and informing regulatory decisions.
What types of research initiatives does bioaccess® specialize in?
Bioaccess® specializes in managing various research initiatives, including Early-Feasibility Assessments (EFS), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF).
What is the regulatory landscape like in Peru for medical technology?
The regulatory landscape in Peru is favorable, characterized by efficient approval processes and government support for innovation, which emphasizes the significance of post-market study design.
How does leveraging real-world evidence from post-market analyses benefit firms?
Leveraging real-world evidence from post-market analyses helps firms navigate the complexities of the healthcare environment effectively and ensures the ongoing safety and efficacy of medical tools.
What role does post-market study design play in improving patient outcomes?
Post-market study design provides insights that can drive product enhancements and inform regulatory decisions, ultimately improving patient outcomes and advancing medical technology in the region.




