Introduction
The Premarket Approval (PMA) process, governed by the FDA, is a pivotal pathway for the introduction of high-risk medical devices into the market. This rigorous procedure demands that manufacturers provide substantial evidence of their products' safety and efficacy, typically through comprehensive clinical trials.
Navigating the complexities of the PMA process is essential for organizations aiming to secure approval and ensure compliance with regulatory standards. With a focus on extensive documentation, including clinical data and manufacturing details, the PMA process not only safeguards public health but also poses significant challenges for medical device startups.
As the landscape evolves, understanding the intricacies of this approval process becomes increasingly vital for stakeholders in the medical technology sector.
Understanding the Premarket Approval (PMA) Process
The PMA process, established by the FDA, serves as a vital regulatory route for high-risk medical equipment. This procedure requires that manufacturers provide significant proof of their product's safety and effectiveness, usually through rigorous clinical trials. A thorough understanding of the PMA process is essential, as it lays the groundwork for all subsequent steps necessary for securing approval.
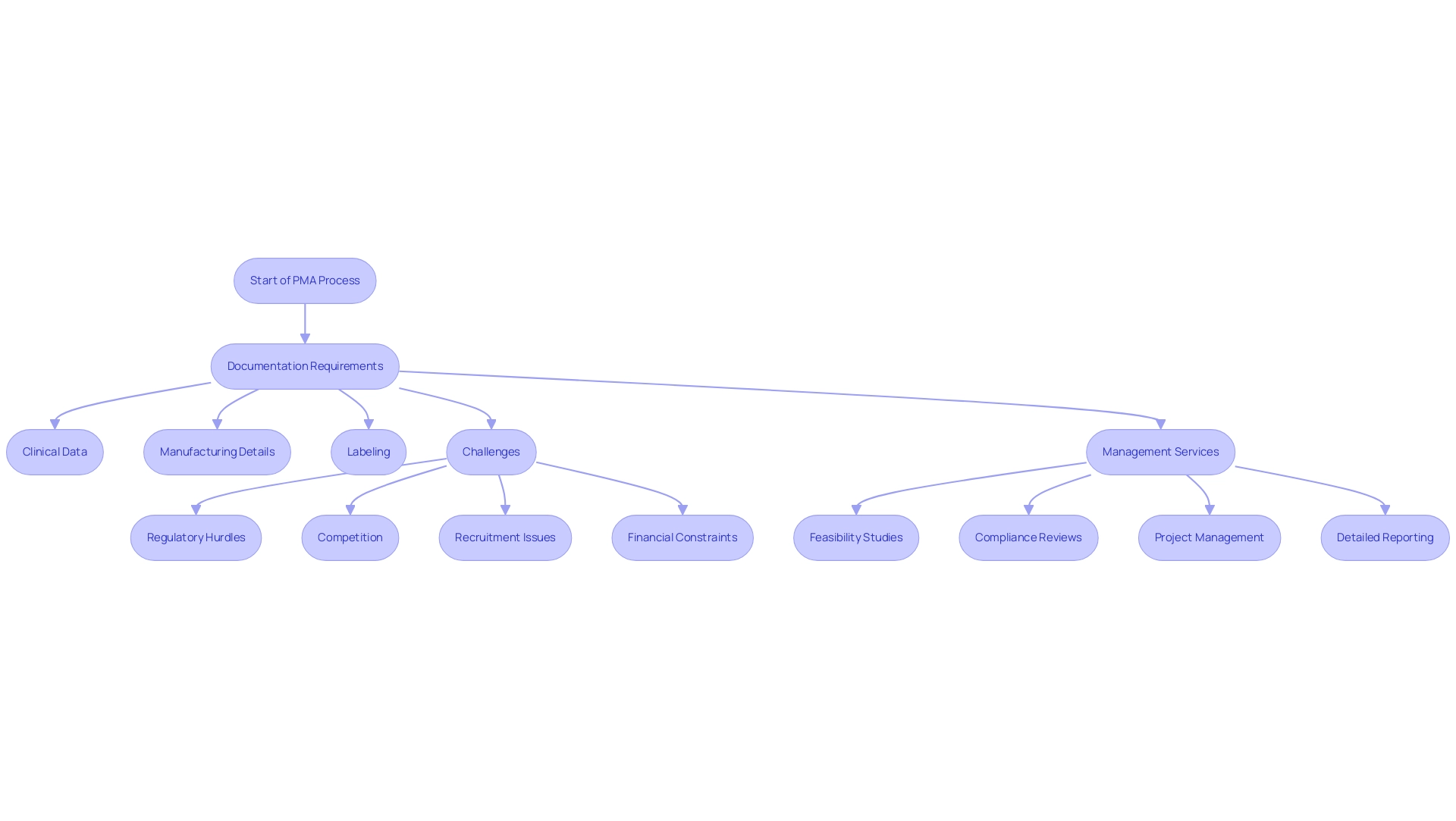
The PMA process includes extensive documentation requirements, such as:
- Clinical data
- Manufacturing details
- Labeling
These requirements ensure that all products comply with established standards before market entry. Recent findings indicate that while 75.4% of respondents believe the scientific evidence is generally adequate for decision-making, many expressed concerns regarding the availability of information on adverse events and long-term outcomes. Furthermore, challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints can complicate the path for medical equipment startups navigating clinical trials.
Furthermore, 26.3% of respondents voiced apprehension that an executive session could extend the timeline, emphasizing potential challenges within the PMA framework. This feedback aligns with the sentiment from recent studies, where panelists highlighted the importance of improved study designs and the necessity for more relevant clinical data. As one panelist noted, data on very old predicate items is often useless and irrelevant.
By utilizing comprehensive clinical trial management services, including:
- Feasibility studies
- Compliance reviews
- Project management
- Detailed reporting on serious and non-serious adverse events
Companies like bioaccess® can assist in the PMA process. Furthermore, the review and feedback on study documents are essential for compliance with country requirements, ensuring that all necessary information is accurately presented. Acquainting oneself with the intricacies of the PMA process, particularly through the knowledge provided by specialized suppliers, will greatly improve your capability to maneuver the complexities linked to medical equipment approval.
Step-by-Step Guide to the PMA Application Process
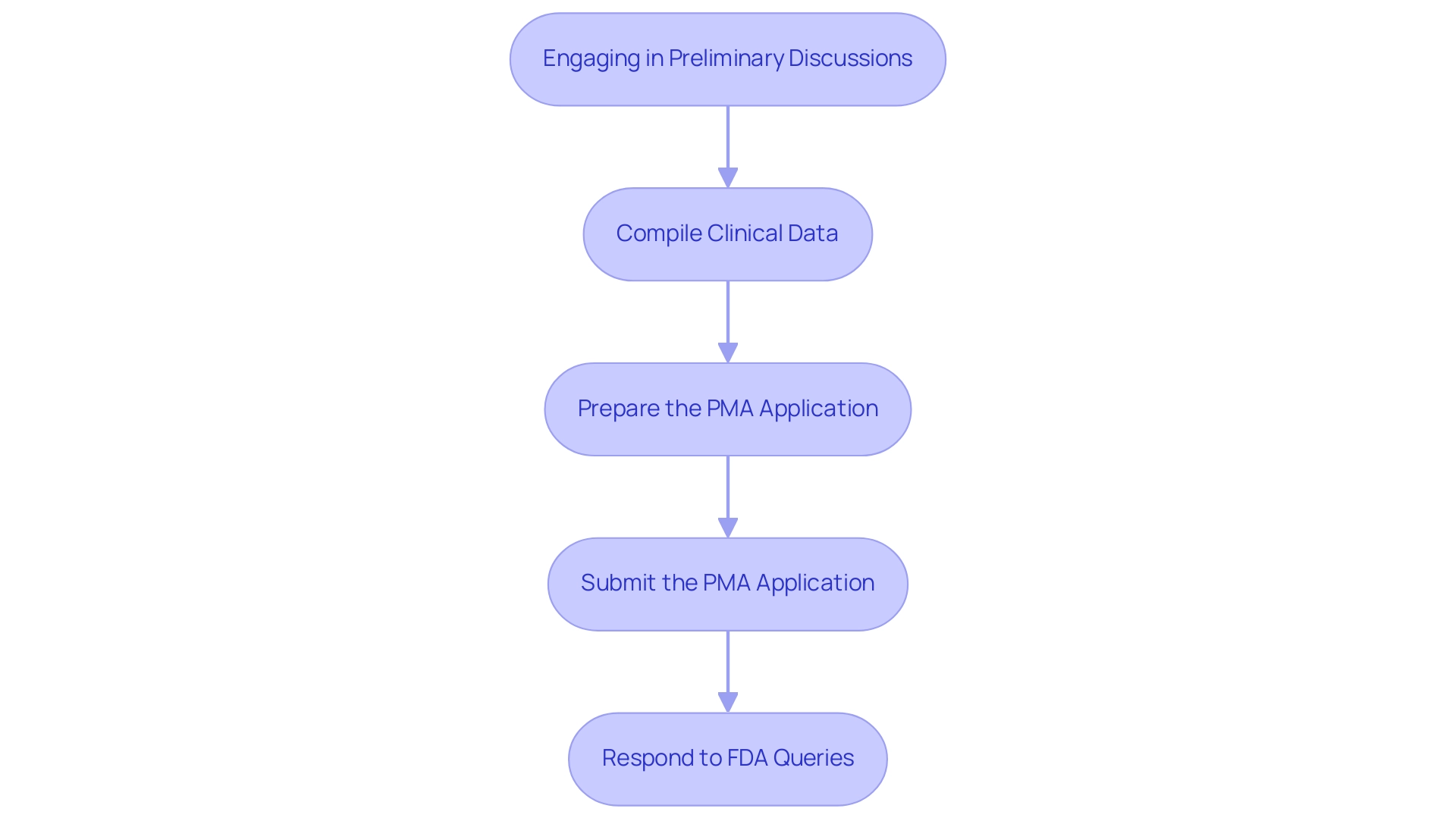
- Engaging in preliminary discussions with the FDA is crucial for clarifying specific requirements and expectations related to the PMA process. This initial step may involve submitting a Pre-Submission (Q-sub) request, which allows for feedback on the proposed study design and clinical data requirements. As noted by Katrina Rogers, readily available data from the FDA informs us we can anticipate a reasonably high (though not 100%) success rate for our PMA and 510(k) medical product submissions.
- Compile Clinical Data: It is essential to gather and analyze robust clinical data that demonstrates both the safety and effectiveness of your product. This process may include conducting clinical trials managed by experts at bioaccess®, who have over 20 years of experience in Medtech and specialize in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF). Our flexibility and specialized knowledge ensure effective data compilation, especially for products classified under the Breakthrough Devices program, which currently includes 124 CDRH items and 4 CBER items authorized. These devices must showcase breakthrough technology, lack approved alternatives, offer significant advantages, or serve the best interests of patients. A notable example is the INSIGHTEC EXABLATE, which successfully obtained marketing authorization, illustrating the PMA process in action.
- Prepare the PMA Application: Organizing your data into the PMA format is a vital step in the PMA process. Ensure that all sections are meticulously completed, including the device description, indications for use, and detailed clinical study reports. Recent changes to FDA pre-submission activities highlight the importance of thorough documentation to streamline the review process.
- Submit the PMA Application: The submission of your PMA application must be conducted through the FDA's electronic submission gateway. It is imperative to ensure that all associated fees are paid, and that all required documents are included in the submission package to avoid delays.
- Respond to FDA Queries: During the FDA's review phase, you should be prepared to respond promptly to any queries or requests for additional information. The FDA intends to request any necessary information within 30 days of receiving the application and will aim to communicate its decision within 60 calendar days. Being proactive in addressing their inquiries can facilitate a smoother review and enhance the likelihood of success. Additionally, bioaccess® provides the expertise and customized approach needed to navigate your company towards acquisition, ensuring that your clinical trials align with business objectives.
Essential Documentation and Requirements for PMA Submission
A successful PMA application hinges on the inclusion of several essential documents, which are supported by comprehensive clinical trial management services. These include:
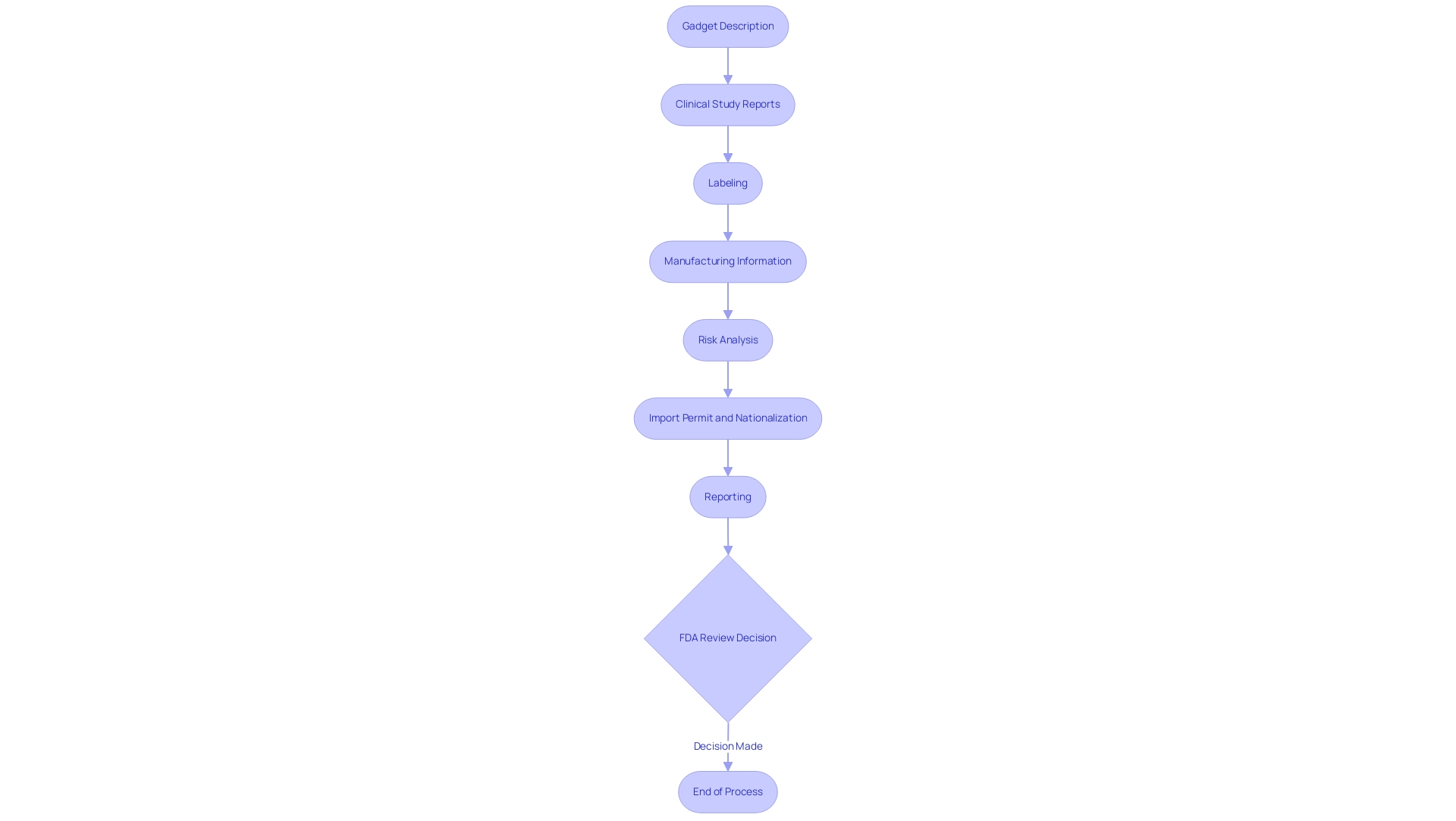
- Gadget Description: A thorough account of the gadget, detailing its design, components, and intended use is vital. This acts as a fundamental component for the FDA’s evaluation framework, improved by adequate feasibility studies and site selection to guarantee adherence to national requirements.
- Clinical Study Reports: Detailed reports from clinical trials are essential for proving the product's safety and effectiveness. These reports should present robust data that supports the claims made regarding the performance of the product and should be carefully monitored throughout the study management process.
- Labeling: Proposed labeling must adhere to FDA requirements, providing clear instructions for use and any necessary warnings. Ensuring adherence to labeling standards is essential, as inaccuracies can result in the rejection of the application, a risk that can be alleviated through expert regulatory review.
- Manufacturing Information: Detailed insights into the production method, quality control measures, and the facilities involved in manufacturing must be included. This information assures the FDA of the reliability and safety of the manufacturing practices, which are essential during trial setup.
- Risk Analysis: Documentation outlining potential risks associated with the product, alongside the mitigation strategies implemented, is required. This analysis is critical in demonstrating that safety considerations have been prioritized throughout the development process and is further supported by project management expertise.
- Import Permit and Nationalization: The application must also include information regarding the import permit and nationalization of investigational items, ensuring compliance with local regulations.
- Reporting: Regular reporting on study status, inventory, and serious and non-serious adverse events is essential to keep all stakeholders informed and to meet regulatory requirements.
As Ann Vankrunkelsven, an RA/QA Manager, aptly notes, the PMA process is the most extensive review process for medical products by the US FDA. They require a complete set of documentation regarding your medical apparatus as well as a well-implemented PMA process. Furthermore, the initial review decision is provided within 45 days after the FDA has received the application, emphasizing the importance of timely and accurate submissions.
The outcomes of a PMA application can vary, as illustrated in the case study titled 'Decision Announcement Outcomes,' where the FDA communicates its decision, which can include an Approval Order, Approvable Letter, Not Approvable Letter, or Order Denying Approval. Each decision type outlines specific actions the applicant can take, such as amending the application or requesting an administrative review. Additionally, if the item contains color additives, these must be listed by the FDA, and a color additive petition may be submitted as part of the PMA.
Thus, ensuring that all documentation is meticulously prepared and well-organized is paramount to the success of the PMA process, significantly reducing the likelihood of rejection. Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, further highlights the significance of maneuvering through these complexities in the Latin American market.
Navigating the FDA Review Process for PMA Applications
The FDA evaluation procedure for Premarket Approval (PMA) applications is a systematic and multi-stage method, crucial for guaranteeing the safety and effectiveness of class III medical instruments within the PMA process. The stages of this procedure are as follows:
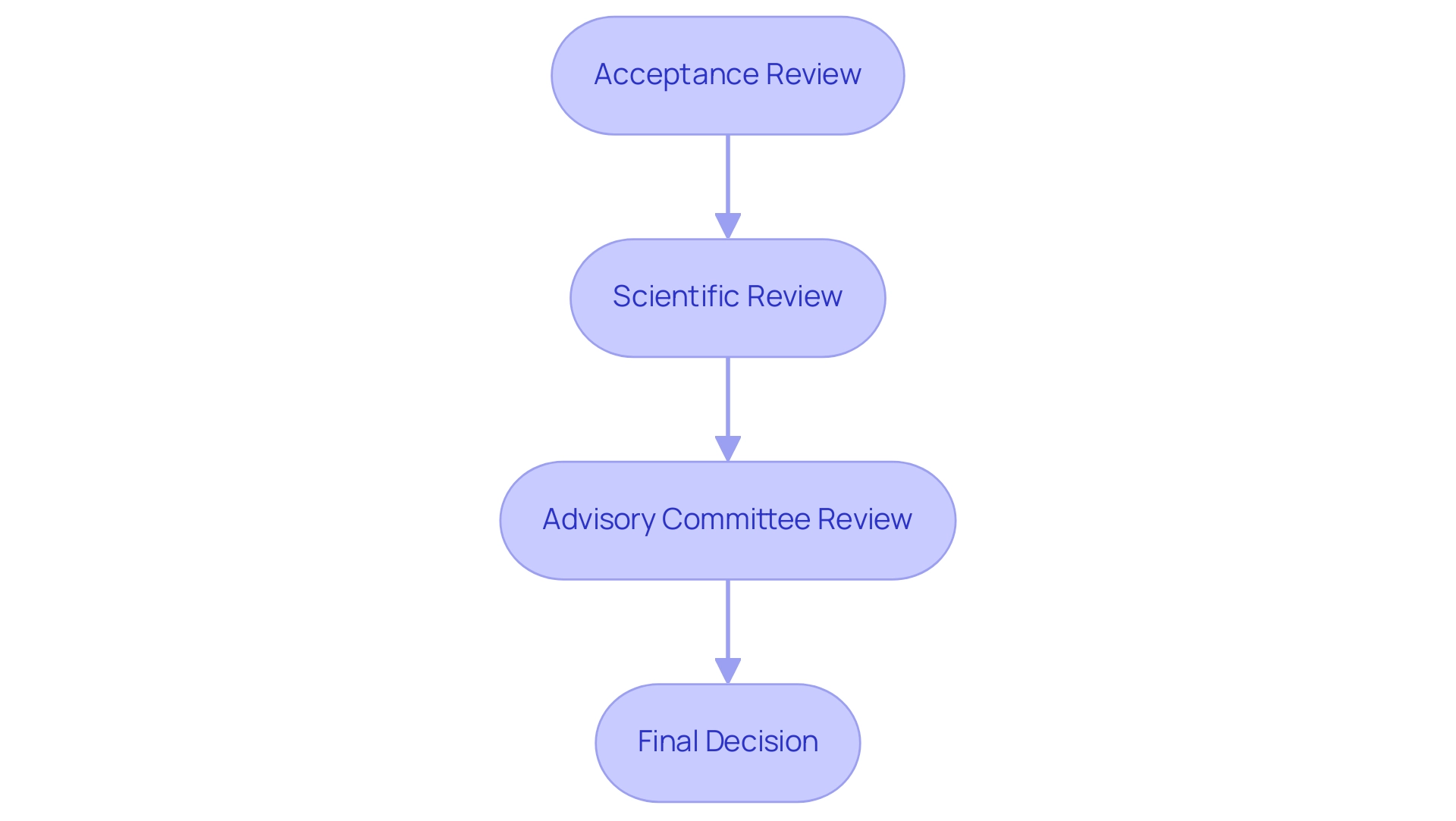
- Acceptance Review: Initially, the FDA evaluates whether the application is complete and suitable for filing. This step is critical as it determines the application’s progression into the review process.
- Scientific Review: During this phase, FDA reviewers rigorously assess the clinical data and supporting documentation. Their goal is to ascertain that the device fulfills the stringent safety and effectiveness criteria established by regulatory standards.
- Advisory Committee Review: In certain situations, the FDA may assemble an advisory committee comprising independent experts to provide insights into the application. Given that a significant portion of the PMA process involves advisory committee input, this stage is crucial for gathering diverse professional perspectives. Panelists have indicated that a three-fourths majority for recommendations would be preferable over the existing voting system, highlighting its significance in the review process.
- Final Decision: Upon completion of the thorough review, the FDA will render a decision to either approve or deny the PMA application. Notably, if the FDA contemplates withdrawing approval, it will issue a notice of opportunity for an informal hearing and publish a Federal Register notice detailing the withdrawal.
When participating in the PMA process, it is vital for manufacturers to consider the advantages of utilizing comprehensive clinical trial management services, such as those offered by bioaccess®. With over 20 years of experience in Medtech, bioaccess® possesses the expertise necessary to navigate the complexities of clinical trials. Their proficiency in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) can streamline trial setup, compliance reviews, and project management, ultimately enhancing the likelihood of a successful PMA process submission.
The PMA process typically takes at least 180 days for FDA approval, which is a critical timeline for manufacturers to consider. Understanding the intricacies of each stage equips manufacturers to better anticipate timelines and effectively prepare for potential obstacles. Furthermore, while most class I medical products and some class II items are exempt from the 510(k) requirements, the simplified self-registration system for these lower-risk products enables faster market entry in contrast to the more stringent PMA procedure.
As noted by Etienne Nichols, Head of Industry Insights & Education at Greenlight Guru, early interaction with the FDA on planned non-clinical and clinical studies and careful consideration of the FDA’s feedback may enhance the quality of subsequent submissions, shorten total review times, and facilitate the development process for new products. This proactive approach can significantly enhance the likelihood of a successful PMA process submission.
Post-Approval Requirements and Compliance for PMA Devices
Upon receiving Premarket Approval (PMA), manufacturers must comply with several critical post-approval requirements as part of the PMA process to ensure the ongoing safety and effectiveness of their medical products, aligning with the comprehensive clinical trial management services offered by bioaccess®, which include Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). These requirements include:
- Post-Market Surveillance: Continuous studies are essential to monitor the product's performance in real-world settings. Manufacturers are obligated to report any adverse events to the FDA promptly, underscoring the regulatory emphasis on patient safety.
- Quality System Regulations (QSR): Adhering to the FDA's quality system regulations is essential to ensure consistent manufacturing practices, thus protecting the quality and reliability of medical products in the market.
- Labeling Updates: Manufacturers must update product labeling as necessary to reflect any new information or changes in indications for use, ensuring healthcare providers have access to the most current data for treatment decisions.
- Periodic Reporting: Regular submission of performance reports to the FDA is required, detailing the outcomes and any issues that may arise, fostering transparency and accountability in management.
The importance of adhering to these regulations cannot be overstated, as non-compliance may jeopardize market access and compromise patient safety. Recent updates in FDA regulations emphasize the need for vigilance; for instance, within 180 days of filing a PMA application, the FDA will issue one of several possible actions, highlighting the regulatory timeline manufacturers must navigate. Additionally, the FDA has established a timeline for Post-Approval Studies (PAS) protocols within the PMA process, mandating completion within 60 days of PMA approval to facilitate timely oversight.
This timely compliance significantly influences patient safety and equipment effectiveness. As noted by expert Etienne Nichols,
Basically, communicating early and often will facilitate the process for both sides and hopefully minimize any delays in what is already a pretty long process,
which stresses the importance of proactive communication in meeting these requirements. Furthermore, ensuring physician awareness of the risks associated with modified medical devices is crucial for overall safety and efficacy.
Staying informed and compliant with these evolving requirements is imperative for manufacturers in 2024 and beyond, particularly with the tailored support from experts like Ana Criado and Katherine Ruiz at bioaccess®.
Conclusion
The Premarket Approval (PMA) process is integral to the introduction of high-risk medical devices, ensuring that products meet stringent safety and efficacy standards mandated by the FDA. This multifaceted procedure requires manufacturers to compile extensive documentation, including clinical data and manufacturing practices, which not only safeguards public health but also presents significant challenges, particularly for medical device startups. The critical nature of this process underscores the need for comprehensive understanding and meticulous preparation at every stage, from initial submission to post-approval compliance.
Navigating the PMA journey involves several key steps, including:
- Pre-submission activities
- Clinical data compilation
- Organization of the PMA application itself
Each phase demands careful attention to detail and robust communication with regulatory bodies to facilitate a smooth review process. The importance of expert support, particularly from clinical trial management services, cannot be overstated, as they provide invaluable insights and assistance in overcoming the complexities tied to the PMA process.
Moreover, post-approval compliance remains a vital aspect of maintaining device safety and effectiveness in the market. Manufacturers are obligated to uphold rigorous monitoring and reporting standards to ensure ongoing adherence to FDA regulations. This commitment to quality not only fosters trust among healthcare providers and patients but also reinforces the integrity of the medical technology sector as a whole.
As the landscape of medical device regulation continues to evolve, stakeholders must prioritize their understanding of the PMA process and the associated requirements. By doing so, they can enhance their chances of successful device approval and contribute positively to the advancement of medical technology, ultimately benefiting public health and safety.
Frequently Asked Questions
What is the PMA process and why is it important?
The PMA (Premarket Approval) process, established by the FDA, is a vital regulatory route for high-risk medical equipment. It requires manufacturers to provide substantial proof of their product's safety and effectiveness, typically through rigorous clinical trials, and lays the groundwork for securing approval.
What documentation is required in the PMA process?
The PMA process includes extensive documentation requirements such as clinical data, manufacturing details, and labeling to ensure compliance with established standards before market entry.
What challenges do medical equipment startups face during the PMA process?
Startups may encounter challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints, which can complicate the path through clinical trials and the PMA process.
What are some common concerns regarding the PMA process?
Concerns include the adequacy of scientific evidence for decision-making, the availability of information on adverse events and long-term outcomes, and potential delays caused by executive sessions.
How can companies improve their chances of navigating the PMA process successfully?
Companies can utilize comprehensive clinical trial management services, including feasibility studies, compliance reviews, project management, and detailed reporting on adverse events to enhance their PMA process.
What is the role of preliminary discussions with the FDA in the PMA process?
Engaging in preliminary discussions helps clarify specific requirements and expectations related to the PMA process, potentially involving a Pre-Submission (Q-sub) request for feedback on study design and clinical data requirements.
What steps should be taken to compile clinical data for the PMA application?
It is essential to gather and analyze robust clinical data demonstrating the product's safety and effectiveness, potentially through clinical trials managed by experts specializing in early-feasibility and post-market studies.
What is involved in preparing the PMA application?
Organizing data into the PMA format is crucial, ensuring all sections are meticulously completed, including device description, indications for use, and detailed clinical study reports.
How is the PMA application submitted to the FDA?
The PMA application must be submitted through the FDA's electronic submission gateway, ensuring all associated fees are paid and all required documents are included to avoid delays.
What should companies do during the FDA review phase of the PMA application?
Companies should be prepared to respond promptly to any queries or requests for additional information from the FDA, as they aim to request necessary information within 30 days and communicate their decision within 60 calendar days.




