Overview
Regulatory compliance for medtech trials in Paraguay is paramount, necessitating a comprehensive understanding of key regulations and active engagement with local experts. Adhering to the ethical standards established by the National Directorate of Drugs and Health Products (DINAVISA) is crucial. This article underscores that thorough preparation—including the compilation of essential documentation and staying abreast of regulatory changes—is vital for effectively navigating the compliance landscape and executing clinical studies. By recognizing these critical factors, stakeholders can enhance their operational success in the evolving medtech environment.
Introduction
In the dynamic world of medical technology, understanding the regulatory landscape is essential for successfully conducting clinical trials, particularly in emerging markets like Paraguay. With its unique set of rules and guidelines governed by the National Directorate of Drugs and Health Products (DINAVISA), navigating this terrain can be both daunting and rewarding.
Comprehending key regulations, ethical considerations, preparing comprehensive documentation, and implementing effective recruitment strategies are crucial for ensuring compliance and advancing medical innovations. As Paraguay positions itself as a burgeoning hub for early-feasibility and first-in-human studies, mastering these elements is vital for stakeholders aiming to thrive in this competitive environment.
This article delves into the essential steps for regulatory compliance, effective trial execution, and overcoming common challenges, providing a roadmap for success in the Paraguayan Medtech landscape.
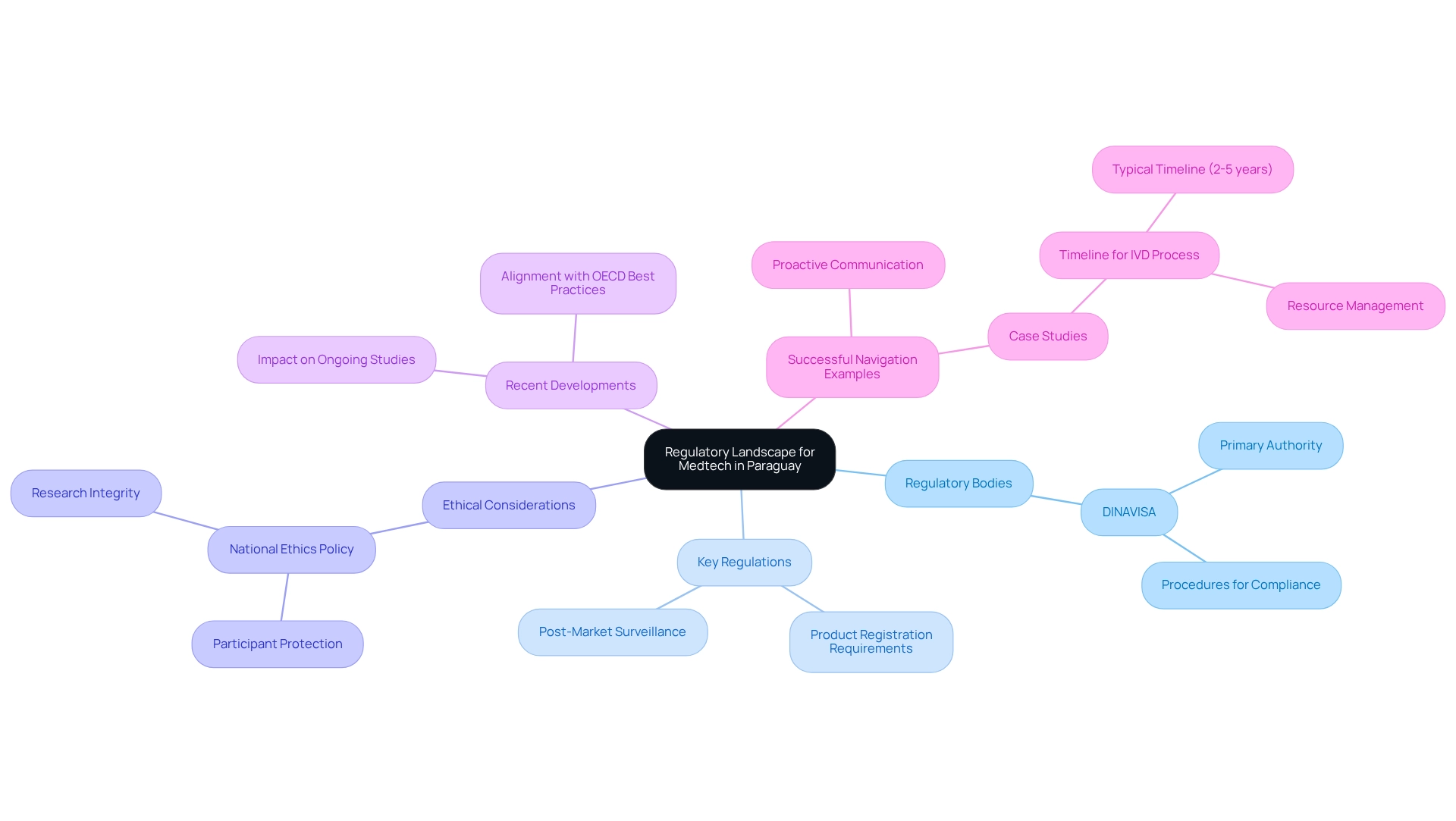
Understand the Regulatory Landscape for Medtech in Paraguay
Navigating the regulatory landscape for Medtech trials in Paraguay demands a comprehensive understanding of several key aspects:
- Regulatory Bodies: The National Directorate of Drugs and Health Products (DINAVISA) stands as the primary authority for medical device regulations. Familiarity with DINAVISA's procedures is essential for achieving regulatory compliance for medtech trials in Paraguay and the successful execution of studies.
- Key regulations highlight that understanding the relevant laws governing medical studies is crucial for ensuring regulatory compliance for medtech trials in Paraguay, including requirements for product registration and post-market surveillance. Staying informed about regulatory compliance for medtech trials in Paraguay facilitates smoother interactions with oversight bodies.
- Ethical Considerations: Adherence to the national ethics policy for health-related research is mandatory. This policy outlines the ethical standards required for conducting medical studies, ensuring the protection of participants and the integrity of the research.
- Staying updated on recent developments is essential for ensuring regulatory compliance for medtech trials in Paraguay. For instance, DINAVISA is currently working to align its clinical trial framework with OECD best practices, which may introduce regulatory compliance for medtech trials in Paraguay that could impact ongoing and future studies. This alignment reflects a broader trend in Latin America, as noted by Julio G. Martinez-Clark, CEO, who stated, "Colombia and Paraguay have become the hottest countries in Latin America for EFS and FIH studies."
- Successful Navigation Examples: Companies that have adeptly navigated DINAVISA regulations often cite thorough preparation and proactive communication with oversight bodies as key strategies. With over 20 years of experience in Medtech, bioaccess® specializes in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Case studies reveal that a clear understanding of the compliance timeline—typically spanning 2-5 years for IVD products—helps stakeholders manage expectations and allocate resources efficiently. The case study titled "Timeline for IVD Process" illustrates this point, emphasizing the importance of understanding the regulatory timeline for effective resource management.
By mastering these elements and leveraging the expertise of bioaccess®, you will enhance your capability to plan and execute research studies in accordance with Paraguayan regulations. Engage with bioaccess® today to ensure your clinical trials are compliant and successful, ultimately contributing to the advancement of medical technologies in the region.
Prepare for Regulatory Compliance: Key Steps and Documentation
To ensure regulatory compliance in Paraguay, it is crucial to adhere to the following essential steps:
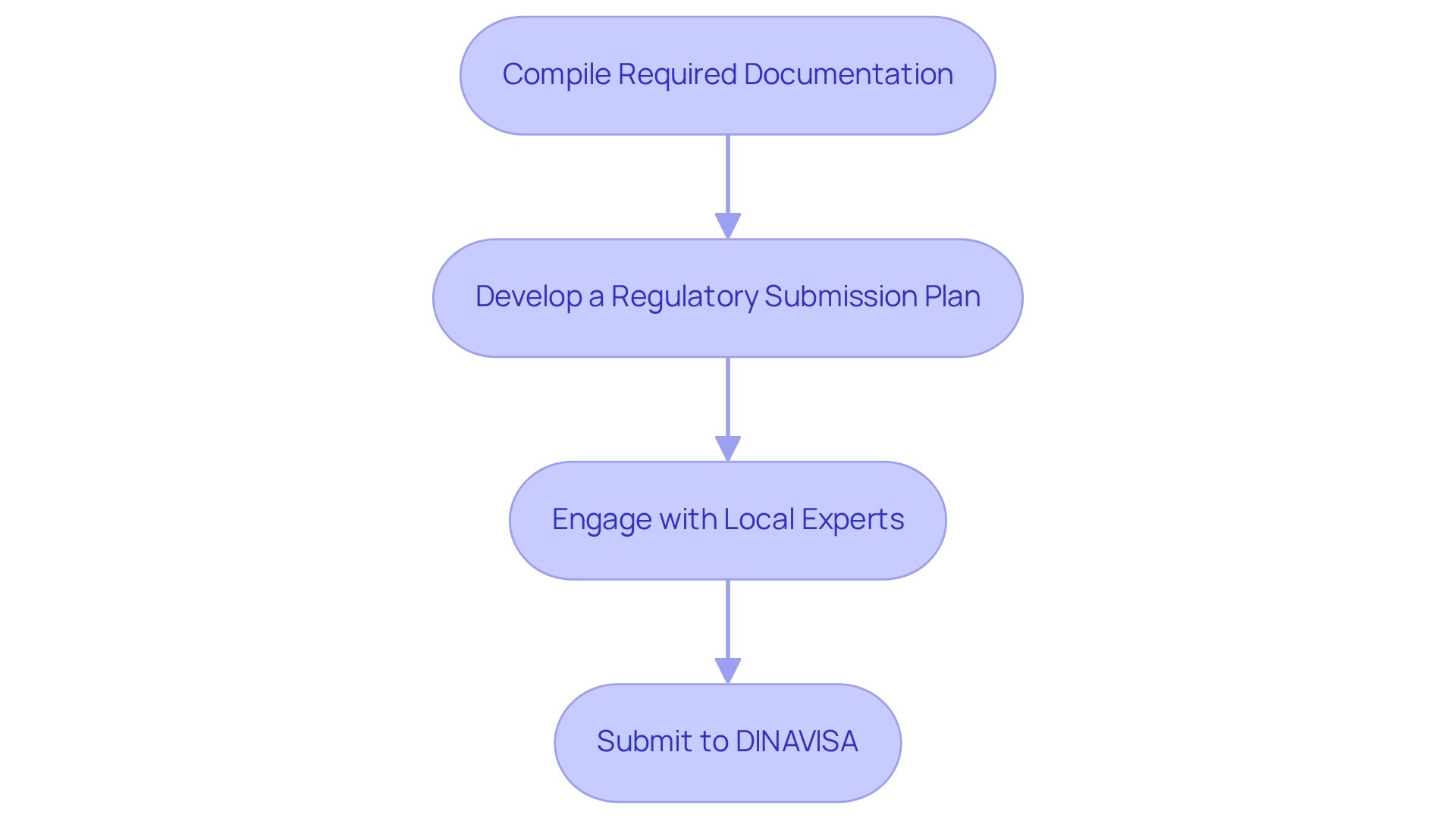
- Compile Required Documentation: Assemble critical documents, including the clinical investigation plan, informed consent forms, investigator brochures, and quality management system documentation, ensuring ISO 13485 compliance. Maintaining high standards in medical device manufacturing requires regulatory compliance for medtech trials in Paraguay.
- Develop a Regulatory Submission Plan: Create a detailed timeline for submission and approval, highlighting key milestones for document preparation and review. This organized approach assists in managing expectations throughout the compliance process. Notably, the timeline for introducing an IVD product to market typically spans 2-5 years, encompassing R&D, validation, and compliance review phases.
- Engage with Local Experts: Collaborate with local specialists or legal advisors who possess a deep understanding of Paraguayan regulations. Their insights can be invaluable in navigating the complexities of regulatory compliance for medtech trials in Paraguay. As emphasized by Julio G. Martinez-Clark, CEO, "The aim is to encourage international collaboration in clinical research and streamline procedures for conducting clinical trials."
- Submit to DINAVISA: Ensure that all documentation is submitted to DINAVISA following their specific guidelines. Be prepared to address any inquiries or requests for additional information promptly.
By thoroughly preparing your documentation and submission approach, you can significantly enhance your likelihood of a successful assessment. Colombia and Paraguay are emerging as frontrunners in early-feasibility and first-in-human studies, indicating an increasing trust in the framework that promotes innovative medical technologies in the region. This framework instills assurance in the quality and performance of IVD products utilized in the Paraguayan healthcare system, emphasizing the importance of regulatory compliance for medtech trials in Paraguay. Furthermore, bioaccess® offers extensive services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring a streamlined process for advancing medical device evaluations.
Execute Clinical Trials: Strategies for Recruitment and Timeline Management
To effectively carry out clinical studies in Paraguay, it is essential to focus on regulatory compliance for medtech trials in Paraguay while implementing the following strategies:
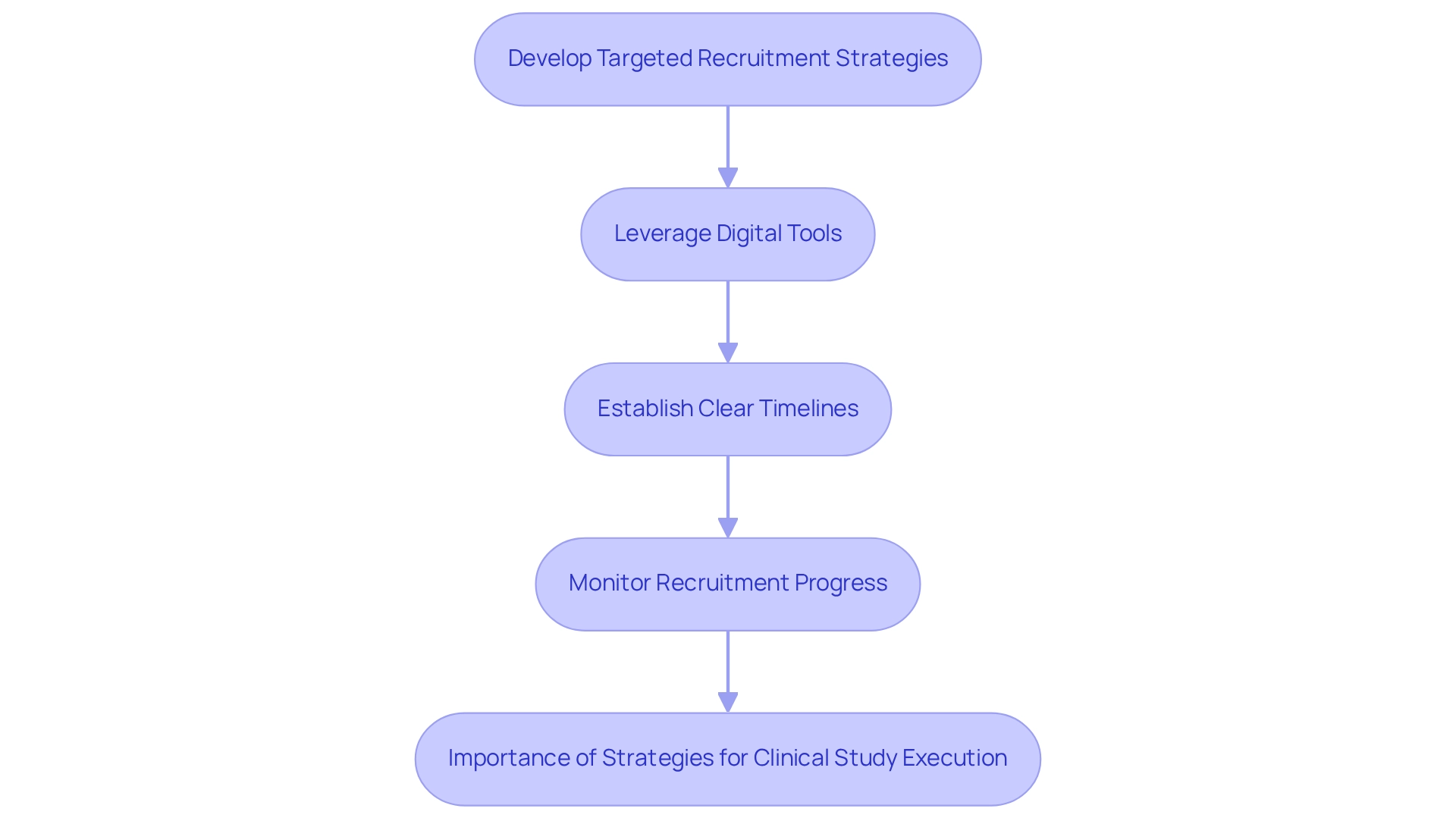
- Develop Targeted Recruitment Strategies: Engage local networks and community outreach initiatives to raise awareness about the study. Adapt your messaging to align with the particular needs and worries of prospective participants, ensuring that they comprehend the study's importance to their health.
- Leverage Digital Tools: Utilize social media and online platforms to broaden your reach and enhance participant engagement. Digital tools have proven effective in improving recruitment rates, allowing for real-time interaction and information dissemination. A study on decentralized clinical studies emphasized the potential for better patient recruitment through innovative technologies, highlighting the significance of incorporating solutions that can simplify processes and improve participant experience.
- Establish Clear Timelines: Create a comprehensive project timeline that outlines all phases of the study, from recruitment through data collection and analysis. Consistently assess and modify these schedules to guarantee that the experiment stays on course and achieves its goals.
- Monitor Recruitment Progress: Implement a robust system for tracking recruitment metrics. This will allow you to recognize any deficiencies early and modify your strategies as needed, ensuring a consistent influx of participants during the study. As mentioned, patient recruitment services aid in the enrollment of participants in clinical studies, accelerating the drug development process and providing quicker access to potentially life-saving treatments for individuals with chronic conditions.
By utilizing these strategies, you can greatly improve participant recruitment and ensure that your clinical study advances smoothly and effectively, ultimately contributing to the progress of medical technologies in the area. Furthermore, it is crucial to consider the necessity for additional research and regulatory compliance for medtech trials in Paraguay to effectively utilize digital technologies for enhancing diversity and equity in studies.
Navigate Challenges: Troubleshooting Common Compliance Issues
Navigating regulatory compliance for medtech trials in Paraguay requires a strategic approach. To enhance your compliance efforts in Paraguay, consider the following essential troubleshooting tips:
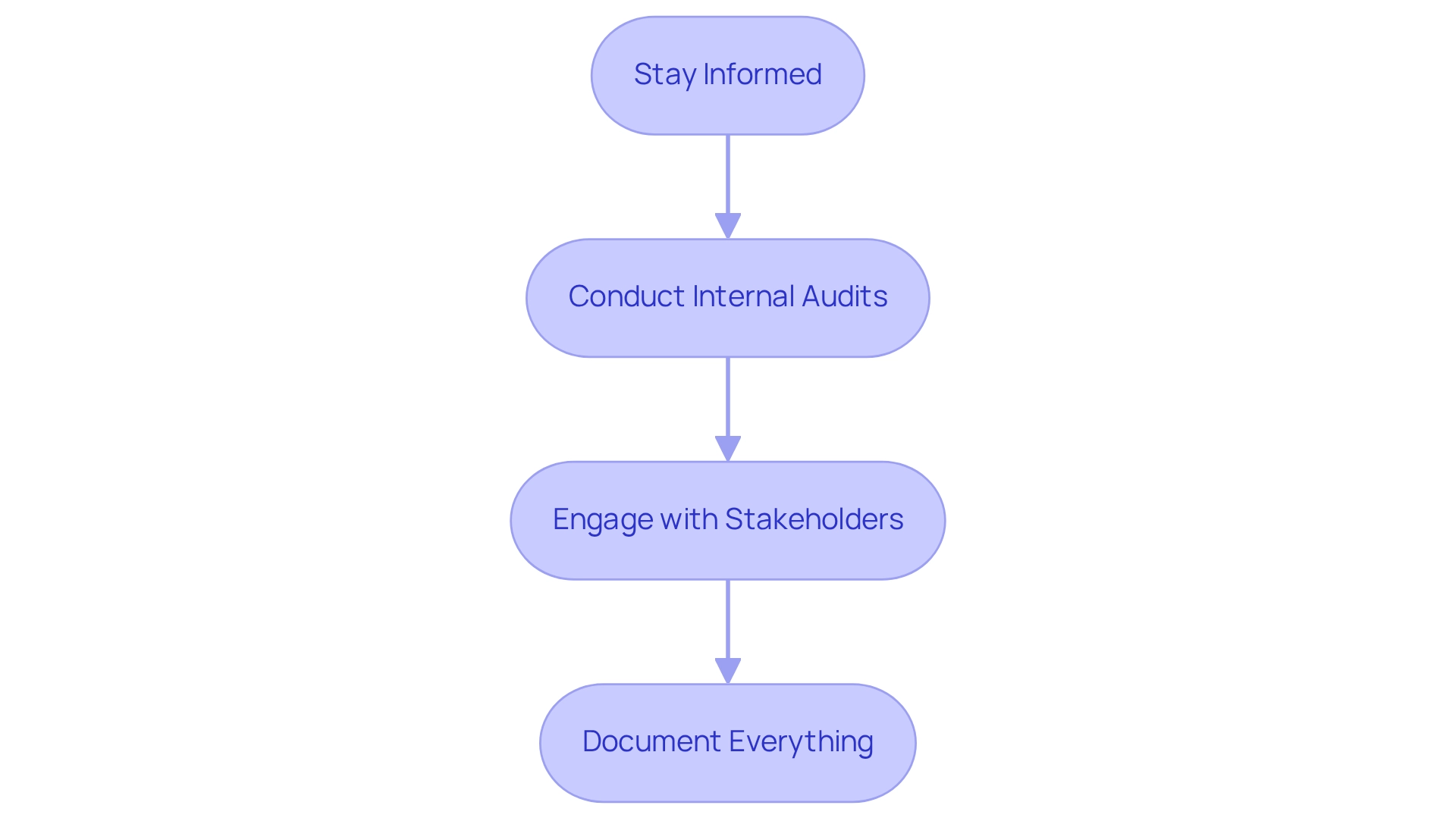
- Stay informed on regulatory compliance for medtech trials in Paraguay by regularly monitoring updates from DINAVISA and other governing bodies to ensure adherence to evolving requirements. This vigilance is crucial, as oversight review periods for medical device studies can range from 90 to 120 days in Colombia, underscoring the importance of timely information. As Julio G. Martinez-Clark, CEO, noted, "Colombia appears to be the sole nation in Latin America actively marketing itself as a premier clinical research destination in the region," highlighting the competitive landscape in which Paraguay operates. Leveraging the expertise of a prominent contract research organization such as bioaccess® can provide significant insights into these legal changes and assist in optimizing your adherence efforts, including feasibility studies and trial preparation.
- Conduct Internal Audits: Implementing routine internal audits is vital for assessing compliance with legal standards. These audits help identify compliance gaps and areas for improvement, fostering a culture of accountability within your organization. Bioaccess® emphasizes the importance of comprehensive project oversight and evaluation to ensure that all aspects of the study align with compliance standards.
- Engage with Stakeholders: Establishing open lines of communication with all stakeholders—including regulatory bodies, ethics committees, and study participants—ensures that concerns are addressed promptly. This collaborative approach can significantly enhance participant involvement and adherence throughout the study process. Consistently evaluating progress and adjusting strategies is essential for maintaining this engagement. Bioaccess®'s extensive research management services encompass stakeholder engagement strategies that can facilitate this process.
- Document Everything: Maintaining thorough records of all communications, submissions, and adherence efforts is essential. A clear audit trail not only fosters transparency but also prepares your organization for any inquiries or audits that may arise. Bioaccess®'s focus on reporting and documentation can assist your organization in preserving these critical records.
By proactively addressing these compliance challenges, you can mitigate risks and enhance the overall success of your clinical trials, ensuring regulatory compliance for medtech trials in Paraguay.
Conclusion
Successfully navigating the regulatory landscape for Medtech trials in Paraguay is paramount for stakeholders aiming to make a significant impact in the medical technology field. A comprehensive understanding of key regulations, ethical standards, and the documentation process is essential for ensuring compliance and advancing innovative solutions. Engaging with local regulatory experts and maintaining open communication with authorities can facilitate smoother interactions and enhance the likelihood of successful trial execution.
Moreover, effective recruitment strategies and the integration of digital tools can significantly streamline participant engagement, ensuring that trials progress efficiently. By establishing clear timelines and monitoring recruitment metrics, stakeholders can address potential challenges proactively, thereby optimizing the clinical trial process.
Ultimately, as Paraguay emerges as a competitive hub for early-feasibility and first-in-human studies, mastering these elements will not only enhance individual trial outcomes but also contribute to the broader advancement of medical technologies in the region. Embracing the guidance of experienced organizations like bioaccess® can provide valuable support in navigating this complex landscape, ensuring that clinical trials are not only compliant but also successful in driving medical innovations forward.
Frequently Asked Questions
What is the primary regulatory body for medical device regulations in Paraguay?
The primary regulatory body for medical device regulations in Paraguay is the National Directorate of Drugs and Health Products (DINAVISA).
Why is it important to understand the relevant laws governing medical studies in Paraguay?
Understanding the relevant laws is crucial for ensuring regulatory compliance for medtech trials, including requirements for product registration and post-market surveillance, which facilitates smoother interactions with oversight bodies.
What ethical considerations must be adhered to in medtech trials in Paraguay?
Adherence to the national ethics policy for health-related research is mandatory, which outlines the ethical standards required for conducting medical studies to protect participants and ensure research integrity.
How is DINAVISA aligning its clinical trial framework, and why is it significant?
DINAVISA is working to align its clinical trial framework with OECD best practices, which may introduce new regulatory compliance measures that could impact ongoing and future studies in Paraguay.
What strategies do successful companies use to navigate DINAVISA regulations?
Successful companies often cite thorough preparation and proactive communication with oversight bodies as key strategies for navigating DINAVISA regulations effectively.
What types of studies does bioaccess® specialize in managing?
Bioaccess® specializes in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What is the typical compliance timeline for IVD products in Paraguay?
The compliance timeline for IVD products typically spans 2-5 years, which helps stakeholders manage expectations and allocate resources efficiently.
How can engaging with bioaccess® benefit clinical trials in Paraguay?
Engaging with bioaccess® enhances the capability to plan and execute research studies in accordance with Paraguayan regulations, ensuring compliance and contributing to the advancement of medical technologies in the region.




