Overview
The article emphasizes the critical role of key players in Latin American clinical trials, illustrating the region's emergence as a significant hub for research, driven by its diverse patient demographics and evolving healthcare infrastructure. It highlights the necessity of collaboration among stakeholders, regulatory bodies, and local expertise to effectively navigate challenges and capitalize on opportunities. This collaborative approach ultimately enhances the efficiency and effectiveness of clinical studies in Latin America, underscoring the importance of a unified effort to advance clinical research in this dynamic landscape.
Introduction
Latin America is emerging as a vital hub in the global clinical trials landscape, driven by a rich tapestry of patient demographics and an evolving healthcare infrastructure. With Brazil leading the charge and significant contributions from Argentina and Mexico, the region has captured 2.1% of the global market revenue, illustrating its growing importance.
However, the journey is not without its hurdles; challenges such as fragmented resources and regulatory complexities pose obstacles to seamless collaboration between local hospitals and international stakeholders.
As innovations in clinical research methodologies take root, partnerships like those between bioaccess™ and Caribbean Health Group are paving the way for Latin America to become a premier destination for clinical trials.
This article delves into the myriad factors shaping the clinical trials environment in Latin America, from cultural and ethical considerations to regulatory dynamics and emerging trends, highlighting both the constraints and opportunities that lie ahead.
Overview of the Clinical Trials Landscape in Latin America
As of April 2024, South America has solidified its role as a pivotal player in the global research studies arena, with Brazil at the forefront, boasting nearly 10,000 registered studies. In 2023, South America accounted for 2.1% of the global research market revenue, underscoring its growing significance. Argentina and Mexico closely follow, further enhancing the region's prominence.
The remarkable diversity in patient demographics across these nations, coupled with an evolving healthcare infrastructure, positions South America as an exceptionally attractive destination for pharmaceutical and biotechnology companies, particularly for major stakeholders in Latin American clinical trials. Nonetheless, challenges such as resource fragmentation and the absence of established contract research organization (CRO) structures impede seamless communication and collaboration between South American hospitals and American research clients. This issue is particularly critical in the first-in-human research sector for medical devices, where professionalism and language barriers further complicate interactions.
Enhanced collaboration with international partners, exemplified by the alliance between bioaccess™ and Caribbean Health Group, aims to establish Barranquilla as a leading hub for medical research in Latin America, supported by Colombia's Minister of Health. Such partnerships foster an environment conducive to innovation in medical methodologies. Notably, while upper middle-income and high-income countries experienced a decline in trial registrations of 4% and 12%, respectively, the rise of CROs—integral players in Latin American clinical trials—has optimized trial management and execution, significantly improving compliance with regulatory standards.
These advancements not only bolster the region's capabilities but also highlight the limitations and opportunities that characterize research in South America. As Gotuzzo from Universidad Peruana Cayetano Heredia articulates, 'Clinical Research in South America: Constraints and Opportunities.' Initiatives like GlobalCare Research Studies, in collaboration with bioaccess™, aim to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates, rapidly transforming the landscape.
Challenges in Conducting Clinical Trials in Latin America
Carrying out medical studies in South America demands navigating a complex array of challenges and opportunities for key players in Latin American clinical trials. As a reliable CRO, bioaccess® plays a crucial role in expediting medical device evaluations throughout the region, where significant variability in regulatory landscapes can lead to extended approval durations, complicating project schedules and heightening uncertainty for stakeholders. Notably, the annual funding in the clinical investigation sector in the Andean Region of South America has surged from $3-4 million to over $50 million, reflecting a growing commitment to development in this field.
Logistical issues further exacerbate these challenges; effective supply chain management and the transportation of investigational products are critical, as delays in these areas can inflate costs and disrupt schedules. Cultural disparities significantly influence the recruitment and retention of participants, making it essential for researchers to engage local investigators, who are key players in Latin American clinical trials and are attuned to these nuances. As highlighted by industry experts, patients recruited overseas often face a 'double disadvantage' due to their underrepresented status and the socioeconomic conditions of their regions.
Leveraging local expertise not only enhances patient engagement but also fosters trust and compliance. Furthermore, stakeholders must remain vigilant regarding the bureaucratic complexities that can arise in these environments, reinforcing the importance of establishing robust partnerships with local institutions to ensure adherence to regulations and to build rapport with study participants. Additionally, with a large, genetically diverse population exceeding 666 million, the region offers significant advantages for research in rare diseases and oncology, providing ample opportunities for biotech companies and researchers to conduct impactful studies.
Addressing these complex challenges is essential for the effective implementation of research studies in this diverse area, positioning bioaccess® as a key player in Latin American clinical trials within the Medtech environment of South America. For any queries or concerns regarding data protection, researchers can contact our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®'), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. Our commitment to transparency and compliance ensures that we address any concerns in accordance with applicable law.
Moreover, our user guides offer thorough instructions on operational methods that further enhance our efficiency in overseeing studies.
Cultural and Ethical Considerations in Clinical Trials
Cultural competence stands as a pivotal element in clinical trials across Latin America. Key stakeholders in these trials must attentively consider the diverse local beliefs, health practices, and social norms that significantly influence study design and participant engagement. Ethical considerations are paramount, particularly concerning informed consent; it is essential that participants fully comprehend the implications of their involvement in studies. Given the historical backdrop of mistrust in medical research, addressing these concerns is crucial for fostering community support and encouraging participation.
This objective can be realized through transparent communication, clarity regarding the process, and a demonstration of potential benefits for the community. Engaging local stakeholders and advocates is instrumental in bridging trust gaps, ultimately enhancing recruitment efforts. Valerie Schaeffer, a consultant at The DOT Connector, LLC, underscores this transition by stating, 'The transition from protocol-focused to patient-focused study design will enhance diverse patient enrollment.'
Such a focus not only honors cultural nuances but also aligns with the broader objective of inclusivity in health research. Our comprehensive research management services encompass feasibility studies, site selection, compliance reviews, setup, and project management, all essential for ensuring that studies are conducted ethically and effectively. The media has increasingly spotlighted research studies in Latin America, providing insights into the landscape and recognizing key players while raising awareness of ongoing investigations.
For instance, Clinical Leader articles accentuate the economic impact of Medtech research studies on local economies, highlighting the role of key players in stimulating job creation, economic expansion, and healthcare enhancement. This significance is further underscored by the statistic that Black Americans constituted only 7% of the Moderna Covid-19 vaccine study, compared to 13% of the US population, illustrating the critical need for diversity in research. Moreover, the case study titled 'Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance' exemplifies how cultural practices can profoundly influence health outcomes and research design.
Maintaining engagement with participants and stakeholders through regular updates, such as weekly or monthly emails, is vital for building trust and ensuring ongoing involvement in research studies.
The Role of Regulatory Bodies in Clinical Trials
Key players in Latin American clinical trials include regulatory bodies such as ANVISA in Brazil and COFEPRIS in Mexico, which play a pivotal role in supervising clinical studies and ensuring adherence to ethical and safety standards. These agencies are responsible for:
- Assessing study protocols
- Monitoring progress
- Enforcing compliance with both local and international regulations
Additionally, INVIMA in Colombia, designated as a Level 4 health authority by PAHO/WHO, supervises the regulation of medical devices and performs comprehensive compliance evaluations essential for successful execution.
The landscape of these regulations is evolving, increasingly shaped by international standards, which has fostered greater collaboration among countries in the region. Such cooperation is essential for streamlining approval processes. Interacting with regulatory bodies from the beginning of the planning process, along with utilizing knowledge from experts such as Katherine Ruiz in Regulatory Affairs for medical devices, is crucial for enabling smoother approvals and effective implementation of clinical studies.
Essential procedural steps encompass:
- Setup
- Start-up
- Acquiring necessary approvals from ethics committees and health ministries
- Obtaining import permits
- Nationalizing investigational devices
Furthermore, robust reporting mechanisms for study status, inventory, and serious and non-serious adverse events are vital for maintaining compliance and transparency throughout the trial. Future paths for medical studies in Latin America highlight the necessity of increasing funding and broadening cancer registries to more effectively tackle the rising cancer burden.
As highlighted in the case study titled 'Scientific Productivity in Oncology,' Brazil, being one of the key players in Latin American clinical trials, leads in scientific output, yet overall productivity remains insufficient to meet this challenge. As Gustavo Gossling aptly notes, 'The academic Cancer Study Groups operating in this region have an essential role in training medical oncologists and specialists in proposing investigator-initiated studies evaluating topics with epidemiological significance for this region.' This underscores the collaborative potential between regulatory bodies and academic institutions in enhancing research productivity.
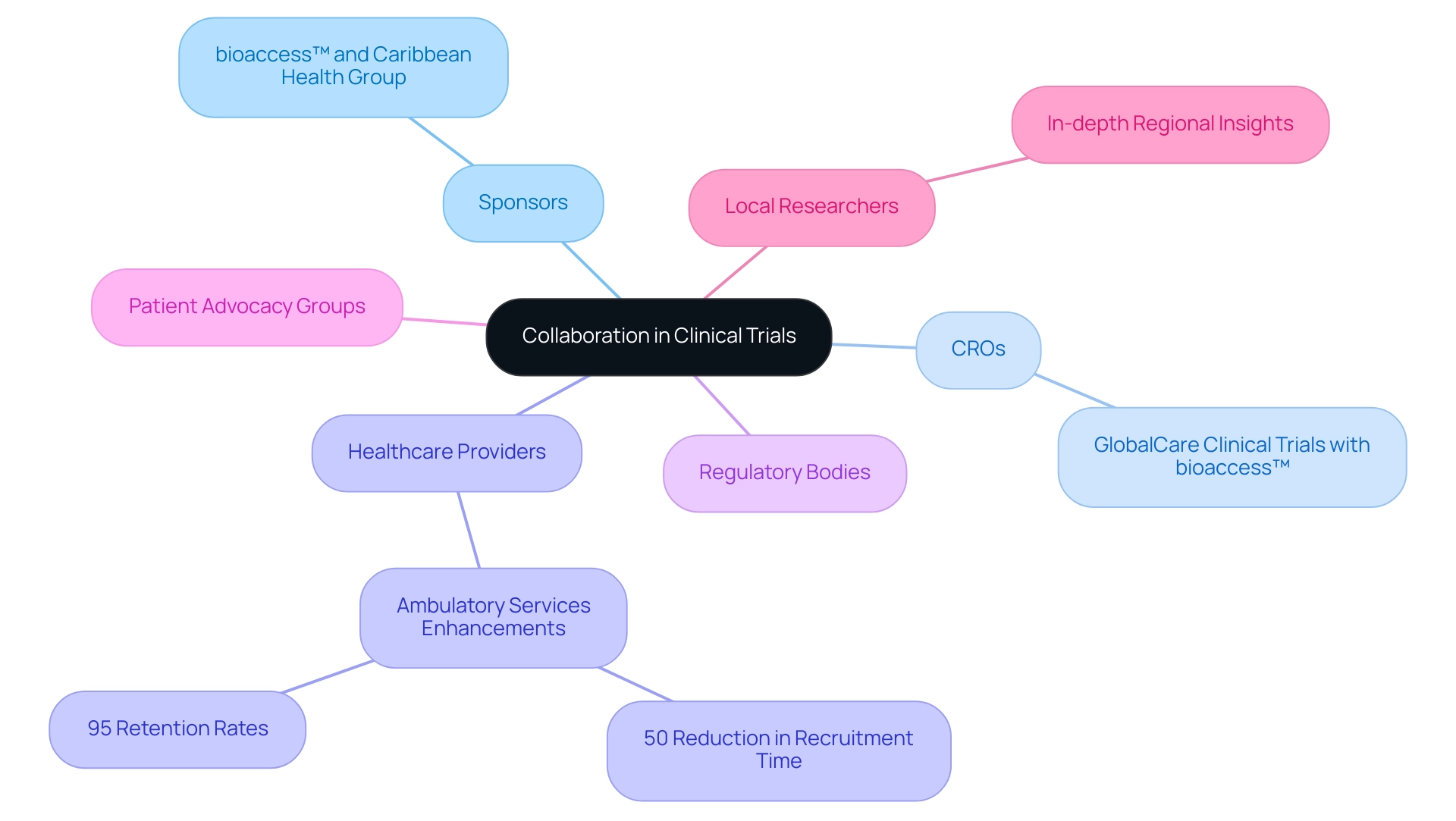
Collaboration Among Key Stakeholders in Clinical Trials
Cooperation among stakeholders—such as sponsors, Contract Research Organizations (CROs), healthcare providers, and regulatory bodies—is vital for the successful implementation of research studies involving key players in Latin American clinical trials. A significant example is the collaboration between bioaccess™ and the Caribbean Health Group, backed by Colombia's Minister of Health, aimed at positioning Barranquilla as a leading destination for clinical studies in the region. Creating strong communication pathways promotes a partnership-oriented atmosphere, greatly enhancing design and implementation processes.
Engaging with local researchers who possess an in-depth understanding of the regional landscape is invaluable, as their insights can enhance recruitment strategies and improve participant retention. Furthermore, collaboration with patient advocacy groups is crucial in addressing ethical concerns, ensuring that study designs prioritize patient needs and perspectives. The involvement of organizations like IDx Technologies, which partners with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection, illustrates how key players in Latin American clinical trials can drive innovative collaborations.
Moreover, GlobalCare Clinical Trials' collaboration with bioaccess™ has established them as key players in Latin American clinical trials, resulting in significant enhancements in ambulatory services in Colombia, achieving over 50% reduction in recruitment time and 95% retention rates. The use of analytical techniques such as the Stakeholder Engagement Assessment Matrix (SEAM) provides a practical framework for assessing stakeholder engagement, facilitating effective communication strategies. As of August 2014, 20 hospitals participated in medical studies, with intentions to include 6 additional ones, highlighting the role of key players in Latin American clinical trials and the significance of partnership in the expanding environment of medical inquiry in the area.
Additionally, a case study named 'Lack of Knowledge or Understanding' demonstrates challenges encountered by organizations, particularly food companies and some investigative organizations, which exhibited a lack of knowledge about the potential of VCTs. This knowledge gap hindered their willingness to explore VCTs, emphasizing the need for better collaboration and information sharing among stakeholders. By leveraging the combined strengths of each stakeholder, the healthcare community can foster a more unified and efficient study environment.
As observed by Professor Philip Kotler, these collaborations are essential in enhancing our methods and improving results in medical research.
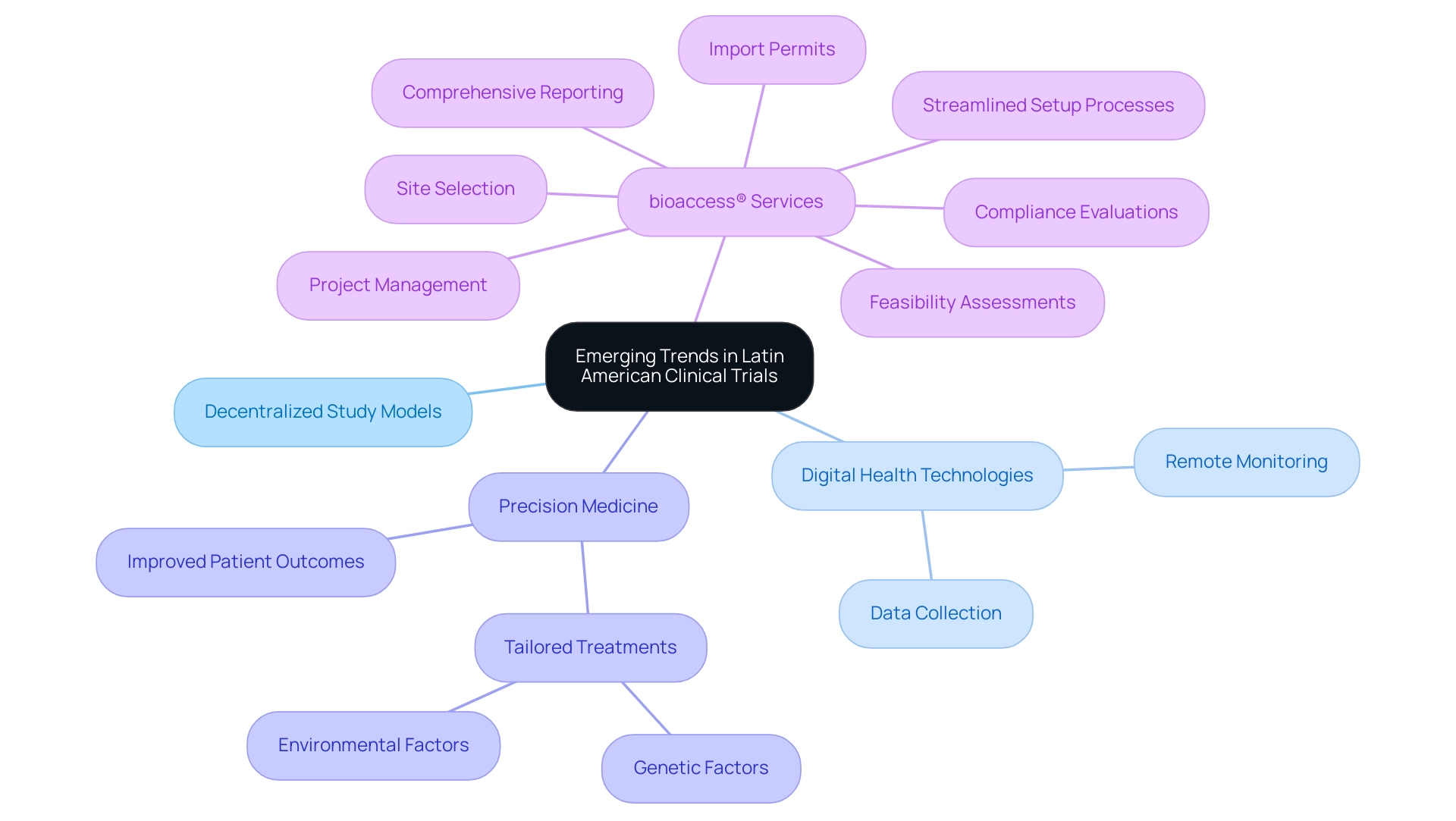
Emerging Trends and Opportunities in Latin American Clinical Trials
Key players in Latin American clinical trials are poised to transform the research landscape in America. Emerging trends, such as the adoption of decentralized study models and an increased reliance on digital health technologies, are at the forefront of this evolution. In 2022, the region experienced over $4 billion in technology investments—more than in the previous decade combined—underscoring its readiness for innovation. These advancements facilitate remote monitoring and data collection, significantly enhancing patient access and participation rates.
Furthermore, the integration of precision medicine is gaining momentum, allowing for tailored treatments that consider genetic and environmental factors. This approach is proving effective in improving patient outcomes. The region is also becoming a focal point for a surge in research studies targeting oncology and rare diseases, driven by the urgent need for new therapies. As emphasized by Gotuzzo from Universidad Peruana Cayetano Heredia in 'Clinical Research in Latin America: Constraints and Opportunities,' key players in Latin American clinical trials must remain agile and ready to leverage these trends to enhance study outcomes and enrich patient experiences.
Significantly, bioaccess® leads the way by providing extensive study management services. These services include:
- Feasibility assessments
- Site selection
- Compliance evaluations to ensure adherence to local regulations
- Streamlined setup processes
- Import permits
- Project management
- Comprehensive reporting
Their expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies positions bioaccess® as a leader in Medtech studies within the region. Moreover, the influence of Medtech research extends beyond experimental settings, aiding local economies through job creation, economic development, and improved healthcare outcomes.
In 2023, Latin America accounted for 2.1% of the global studies market, with expectations for considerable expansion. This growth positions key players in Latin American clinical trials as benchmarks for FSP achievement in the global environment.
Future Directions for Clinical Trials in Latin America
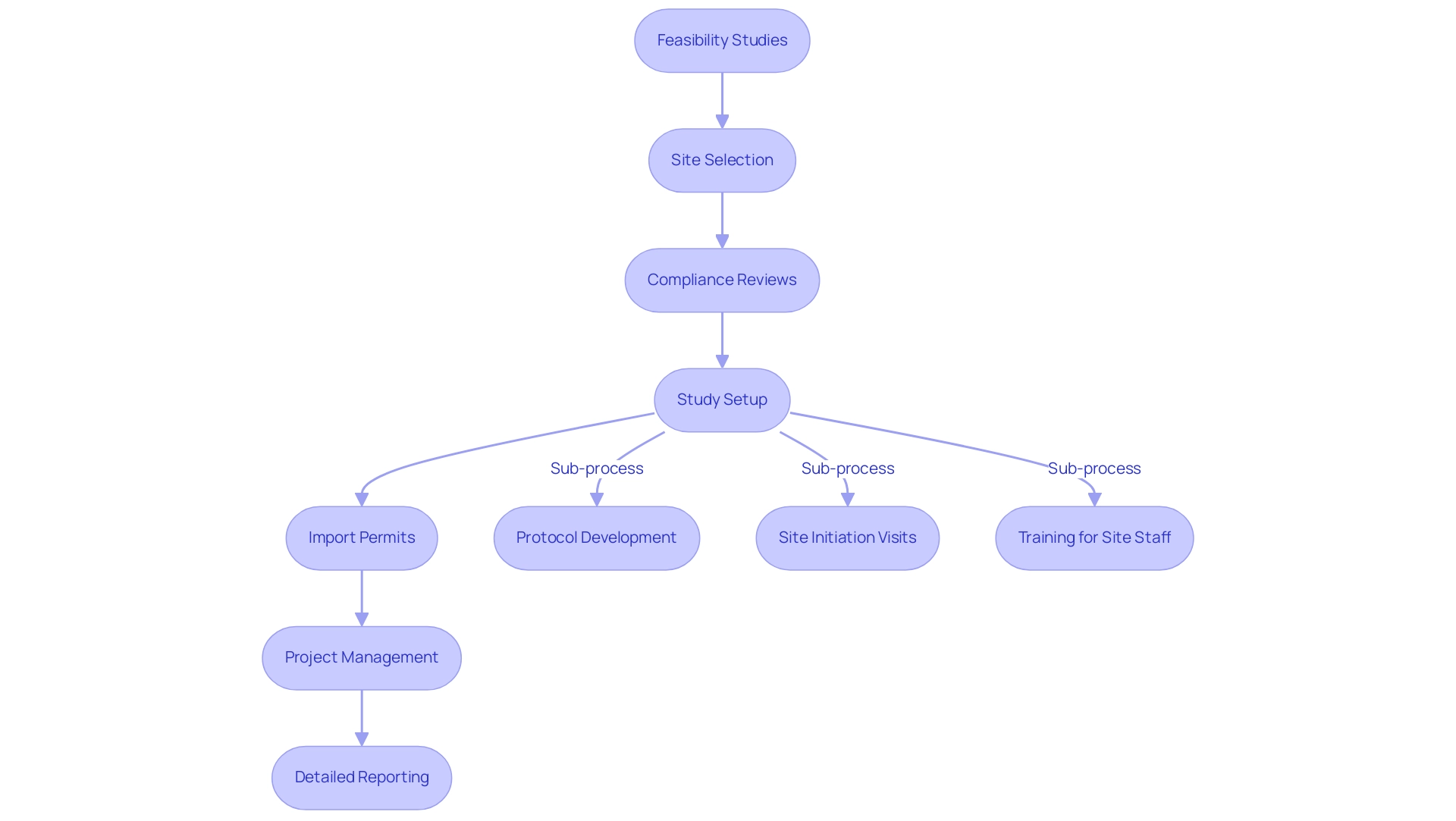
The future of medical studies in Latin America is poised for significant transformation, driven by advancements in technology, regulatory changes, and a renewed emphasis on patient-centered research. Key players in Latin American clinical trials are at the forefront of this evolution. Our comprehensive clinical study management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Detailed reporting
Specifically, our study setup includes:
- Protocol development
- Site initiation visits
- Training for site staff
This ensures that all processes comply with local regulations.
Rigorous monitoring throughout the study is crucial to ensure adherence to protocols and timely reporting of:
- Study status
- Inventory management
- Both serious and non-serious adverse events
As artificial intelligence and machine learning become increasingly integrated into research processes, they promise to streamline data analysis, enhance patient recruitment strategies, and elevate predictive analytics for study outcomes. For instance, AI can assist researchers in identifying eligible participants more efficiently, thus accelerating the recruitment phase.
Regulatory structures are expected to develop, fostering a more unified environment for studies throughout the area, which could lessen obstacles to carrying out investigations. Moreover, the dedication to diversity and inclusion in health studies is gaining momentum, as stakeholders acknowledge the importance of involving underrepresented groups to ensure that results are pertinent and applicable to a broader patient demographic. The economic impact of these studies is noteworthy, as they contribute to job creation and healthcare improvement, fostering international collaboration.
The quantity of scientific publications in South America and the Caribbean has risen from 1,978 in 2015 to 3,410 in 2020, indicating a strong growth in research activity that bolsters the future of medical studies. Furthermore, as mentioned in the case study, the region accounted for 2.1% of the worldwide research market revenue in 2023, emphasizing its present position and potential for expansion in the global market. As highlighted by Gustavo Werutsky, pharmaceutical firms have supported most cancer research efforts in South America and the Caribbean, and additional funding will also be required from government or private organizations.
This appeal for extra funding underscores the essential requirement for joint initiatives to enhance the future of medical studies. By embracing these emerging trends and leveraging local academic cancer research groups, key players in Latin American clinical trials are set to solidify their role as vital contributors to the global clinical trials market, navigating the complexities of research while aligning with international standards and practices.
Conclusion
Latin America stands at the precipice of a transformative era in clinical trials, showcasing its potential as a significant player in the global landscape. With Brazil, Argentina, and Mexico leading the charge, the region has demonstrated a remarkable ability to attract investment and enhance its healthcare infrastructure, contributing to 2.1% of the global clinical trials market in 2023. This growth is fueled by a rich diversity in patient demographics and an increasing number of registered trials, particularly in sectors like oncology and rare diseases.
However, the path forward is not without its challenges. Fragmented resources, regulatory complexities, and cultural considerations can hinder the efficacy of clinical trials. Yet, initiatives aimed at fostering collaboration among stakeholders—including sponsors, CROs, healthcare providers, and regulatory bodies—are paving the way for a more integrated approach. Partnerships like those between bioaccess™ and local health organizations illustrate the importance of leveraging local expertise and building trust within communities, which is essential for successful trial execution.
Looking ahead, the adoption of decentralized clinical trial models and advancements in digital health technologies present exciting opportunities for enhancing patient engagement and streamlining processes. As regulatory frameworks evolve to support these innovations, the potential for Latin America to emerge as a model for clinical research success becomes increasingly tangible. By prioritizing patient-centered research and addressing the unique challenges of the region, Latin America is poised to solidify its role as a vital contributor to the global clinical trials market, ultimately improving healthcare outcomes and fostering economic growth.




