Overview
The article focuses on key innovations and success stories emerging from clinical trials in Latin America, underscoring the region's rapid growth and potential in medical research. It asserts the critical importance of collaboration between industry and academia, innovative methodologies, and a favorable regulatory environment. Together, these elements have significantly enhanced trial efficiency and healthcare outcomes in countries such as:
- Colombia
- Brazil
- Argentina
This collaboration not only fosters advancements in clinical research but also addresses key challenges faced by the Medtech landscape. The insights presented here illustrate the necessity for continued partnership and innovation to sustain this momentum. In conclusion, the importance of collaboration cannot be overstated, as it paves the way for future advancements and improved healthcare outcomes in the region.
Introduction
In recent years, Latin America has emerged as a vibrant hub for clinical trials, showcasing a plethora of success stories that highlight innovative methodologies and strategic collaborations. With investments in clinical research skyrocketing—particularly in countries like Brazil, Argentina, and Mexico—the region is rapidly becoming a focal point for Medtech advancements. This synergy between academia and industry fosters a fertile ground for groundbreaking studies. Moreover, cultural factors and regulatory improvements are paving the way for enhanced patient participation and protection. As stakeholders navigate the unique challenges of this diverse landscape, the transformative potential of Latin America in the global clinical research arena cannot be overstated. This makes it an exciting frontier for future innovations and healthcare breakthroughs.
Introduction to Success Stories in Latin America Trials
The region of America has emerged as a vibrant hub for research studies, showcasing success stories in Latin America trials that highlight innovative methodologies and approaches. Over the last decade, the area has witnessed a remarkable increase in investment, with yearly funding for trials in the Andean Region skyrocketing from $3-4 million to over $50 million. This growth is particularly pronounced in Brazil, Argentina, and Mexico, with significant advancements also observed in Peru, Colombia, and Chile.
Partnerships, such as that between bioaccess™ and Caribbean Health Group, are pivotal in establishing Barranquilla as the most attractive location for medical studies in Latin America, a fact endorsed by Colombia's Minister of Health. The success stories emerging from Latin America trials not only underscore the region's potential but also provide a roadmap for future research initiatives. Notably, the sector has found that:
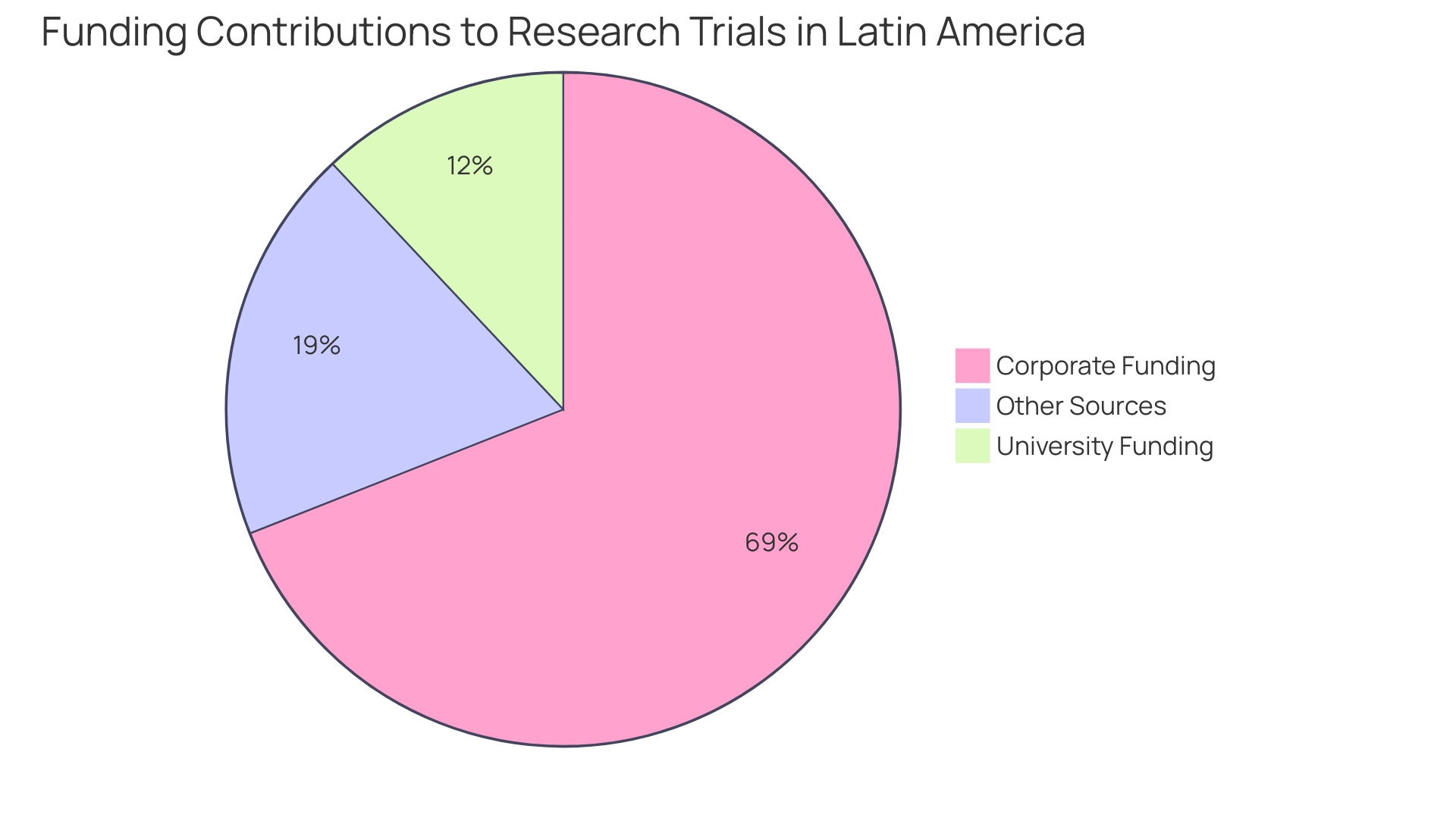
- 69% of research studies are funded by corporations
- Universities contribute 12%
This indicates a robust collaboration between academia and industry. This partnership is essential for fostering innovation and ensuring that studies are designed to meet both scientific and patient needs.
Cultural factors significantly influence patient involvement in research studies. In many cases, patients in Latin America may consent to participate in studies without fully discussing treatment options or risks with their physicians, reflecting distinct cultural attitudes towards medical authority and decision-making. Addressing these cultural nuances is vital for enhancing patient protection and improving the overall quality of medical studies.
Moreover, the necessity for research trials involving pediatric populations remains a pressing concern, as current participation rates are limited. Countries are encouraged to develop incentives for such studies while prioritizing the rights and well-being of vulnerable participants.
As Julio G. Martinez-Clark, CEO, remarked, "Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy." This statement underscores the commitment of South American nations to enhance their research capabilities, further illustrated by collaborations such as that of IDx Technologies with bioaccess™ to identify ophthalmology centers in South America for AI-based disease detection partnerships and GlobalCare Clinical Trials' improvement of ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
Looking ahead, the innovative approaches being adopted can be seen as success stories in Latin America trials that serve as models for other regions. By leveraging local knowledge and addressing the unique challenges posed by linguistic, cultural, and socio-economic barriers, stakeholders can enhance the efficiency of research studies and ultimately improve healthcare outcomes throughout the region.
The Role of First-in-Human Trials in Advancing Medtech
First-in-human (FIH) evaluations are essential in the clinical development landscape, serving as the foundational testing phase for new medical devices and therapies. These studies are critical for assessing safety and efficacy, laying the groundwork for subsequent research phases. In South America, numerous FIH studies have successfully contributed to success stories in Latin America trials, enabling the introduction of innovative medical technologies and underscoring the region's potential to advance global healthcare.
The importance of FIH studies cannot be overstated, particularly within the Medtech sector. Insights from industry leaders, including Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, underscore how bioaccess® has been pivotal in managing the complexities of FIH studies during its inaugural human examination in Colombia. This collaboration demonstrates the expertise bioaccess® provides in managing a range of studies, including:
- Early-Feasibility Studies (EFS)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This highlights its commitment to effectively enhancing medical devices.
As we progress into 2025, the landscape for FIH studies in South America is evolving rapidly, with an increasing emphasis on regulatory readiness. The growing complexity of regulations necessitates that Medtech companies remain vigilant about international guidelines to ensure a seamless commercialization process. Dipanwita Das, CEO & co-founder, emphasizes that adhering to FDA recommendations and staying informed about data privacy laws is crucial for success in this demanding environment.
Recent statistics reveal a significant trend: between 2020 and 2023, high-income nations experienced a 12% decline in study registrations, signifying Latin America's emergence as an Alternative Center for medical studies. The region's diverse patient populations and favorable regulatory environments render it a compelling choice for conducting FIH studies.
A noteworthy legislative advancement is the endorsement of Law 14.874/24 in Brazil in May 2024, which aims to simplify the assessment procedure for studies. This initiative seeks to reduce bureaucratic obstacles, enhancing predictability and ultimately boosting clinical research activities. Consequently, Brazil is poised to become a more attractive location for Medtech companies, facilitating faster access to innovative medical technologies for patients.
The success stories emerging from Latin America trials stemming from FIH studies further illustrate the region's capabilities. These experiments have not only propelled medical device development but have also significantly improved healthcare outcomes and contributed to local economies through job creation and economic growth. By connecting innovative Medtech companies with trial opportunities, bioaccess® is strategically positioned to expedite the advancement of medical devices, solidifying the region's role in the future of medical technology.
Advantages of Conducting Trials in Latin America
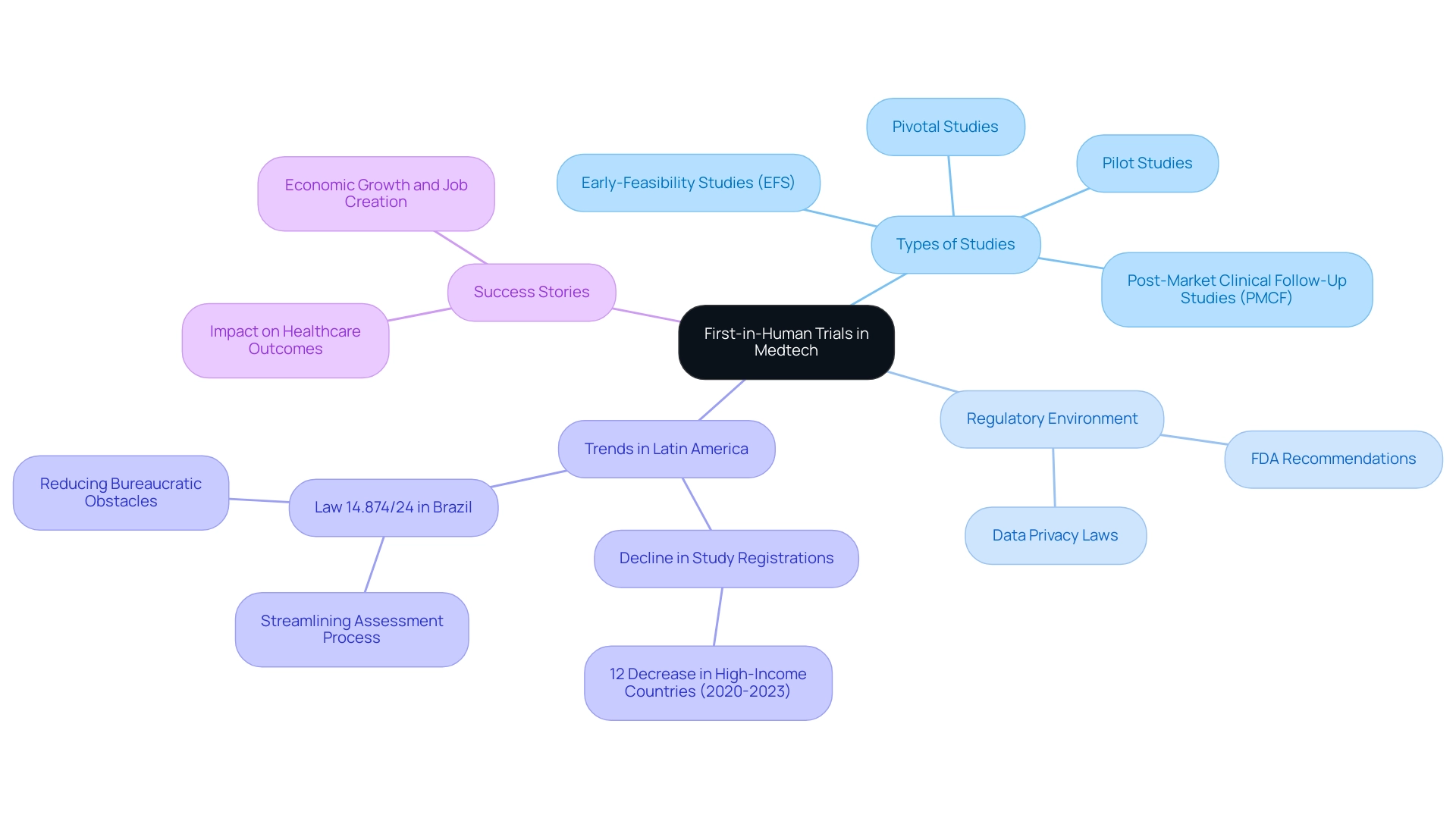
The region of America stands out as a prime location for conducting medical trials, showcasing a multitude of advantages that significantly enhance the research process. Notably, operational costs are considerably lower compared to other regions, leading to substantial savings for Medtech companies. Furthermore, access to diverse patient demographics is essential for ensuring that success stories in Latin America trials accurately reflect a broad spectrum of populations and health conditions.
The genetic diversity found in Latin America, combined with its urbanized demographics, plays a crucial role in facilitating quicker patient recruitment and retention. This is particularly beneficial for Medtech companies aiming to accelerate their clinical studies, underscoring the success stories in Latin America trials. For instance, Colombia has made significant strides by implementing a comprehensive science, technology, and innovation strategy for 2022–2031, aiming to transform the country into a knowledge economy.
This initiative emphasizes collaboration to meet the unique needs of its diverse populations and accelerate the development of new therapies. As Julio G. Martinez-Clark, CEO, states, "Colombia acknowledges the advantages of improving medical studies and has created an ambitious science, technology, and innovation strategy for 2022–2031 aimed at evolving into a knowledge economy."
Moreover, the regulatory environment in Latin America, particularly overseen by INVIMA as a Level 4 health authority by PAHO/WHO, is increasingly streamlined. This is essential for creating success stories in Latin America trials, allowing for faster approvals and a more efficient trial process. The region's commitment to improving healthcare access and diversity in study enrollment enhances this situation, contributing to success stories that align with the global push for inclusive trials. As Allison Kalloo, Founding Partner and Communications Lead of Clinical Ambassador and iParticipate, notes, "The current landscape is shaped by a demand for improved healthcare access and a commitment to diversity in research enrollment."
With over 20 years of experience, bioaccess® offers a personalized approach to managing studies, ensuring that each project is tailored to the specific requirements of Medtech firms. Our comprehensive services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Testing setup
- Import permits
- Project management
- Reporting
This distinctive value proposition connects innovative Medtech firms with opportunities for conducting studies in South America.
Consequently, Medtech firms can leverage these benefits to conduct cost-effective studies that meet regulatory standards while fostering medical exploration in the region, ultimately contributing to job creation and economic development.
In summary, the combination of reduced expenses, diverse patient access, and an evolving regulatory environment positions this region as an appealing choice for Medtech research studies. This leads to success stories in Latin America trials, establishing it as a key player in the global research arena.
Case Studies: Innovations That Transformed Clinical Trials
Success stories from clinical trials in Latin America illustrate the profound impact of innovative methodologies on research studies. A notable advancement is the collaboration between GlobalCare Clinical Trials and bioaccess™ aimed at enhancing ambulatory services in Colombia, achieving over a 50% reduction in recruitment time while maintaining a retention rate exceeding 95%. Additionally, IDx Technologies' partnership with bioaccess™ to identify ophthalmology centers for AI-based disease detection highlights a commitment to harnessing technology for improved health outcomes.
Moreover, bioaccess® specializes in managing a diverse array of medical device research studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
The integration of advanced data analytics has streamlined operations, allowing researchers to make data-driven decisions swiftly. This approach is crucial for navigating the complexities of medical studies, particularly in a diverse region like South America, where logistical challenges often impede progress.
These innovations extend beyond mere operational enhancements; they are essential in driving improved patient outcomes. For instance, a recent study revealed that decentralized designs resulted in a 30% increase in patient retention compared to traditional methods. Such statistics underscore the region's potential to emerge as a leader in medical studies, with success stories from Latin America trials showcasing its unique capabilities to efficiently address global health challenges.
As we approach 2025, the emphasis on groundbreaking innovations in medical studies will continue to shape the landscape, as evidenced by success stories in Latin America trials, establishing the region as a hub for advanced methodologies. Ongoing advancements in study designs and data management practices reflect a commitment to enhancing the research ecosystem, ultimately benefiting both participants and the broader medical community.
Furthermore, the Asia Pacific region is projected to reach USD 12,687.3 million by 2030, indicating significant growth potential for research studies globally. As noted by Matt Stewart, "As we look ahead to 2025, the consumer health market is on the cusp of significant growth, driven by innovation, digital transformation, and a focus on sustainability." This insight highlights the importance of creative approaches in medical studies.
Additionally, the resurgence of Medical Device Regulation (MDR) solutions underscores the necessity for effective metadata management to improve study design and efficiency. The Horizon Databook's support for academic and research non-profit organizations at reduced rates exemplifies the broader support for medical research in the region, further solidifying its role in the international research landscape.
Challenges and Solutions in Latin American Clinical Trials
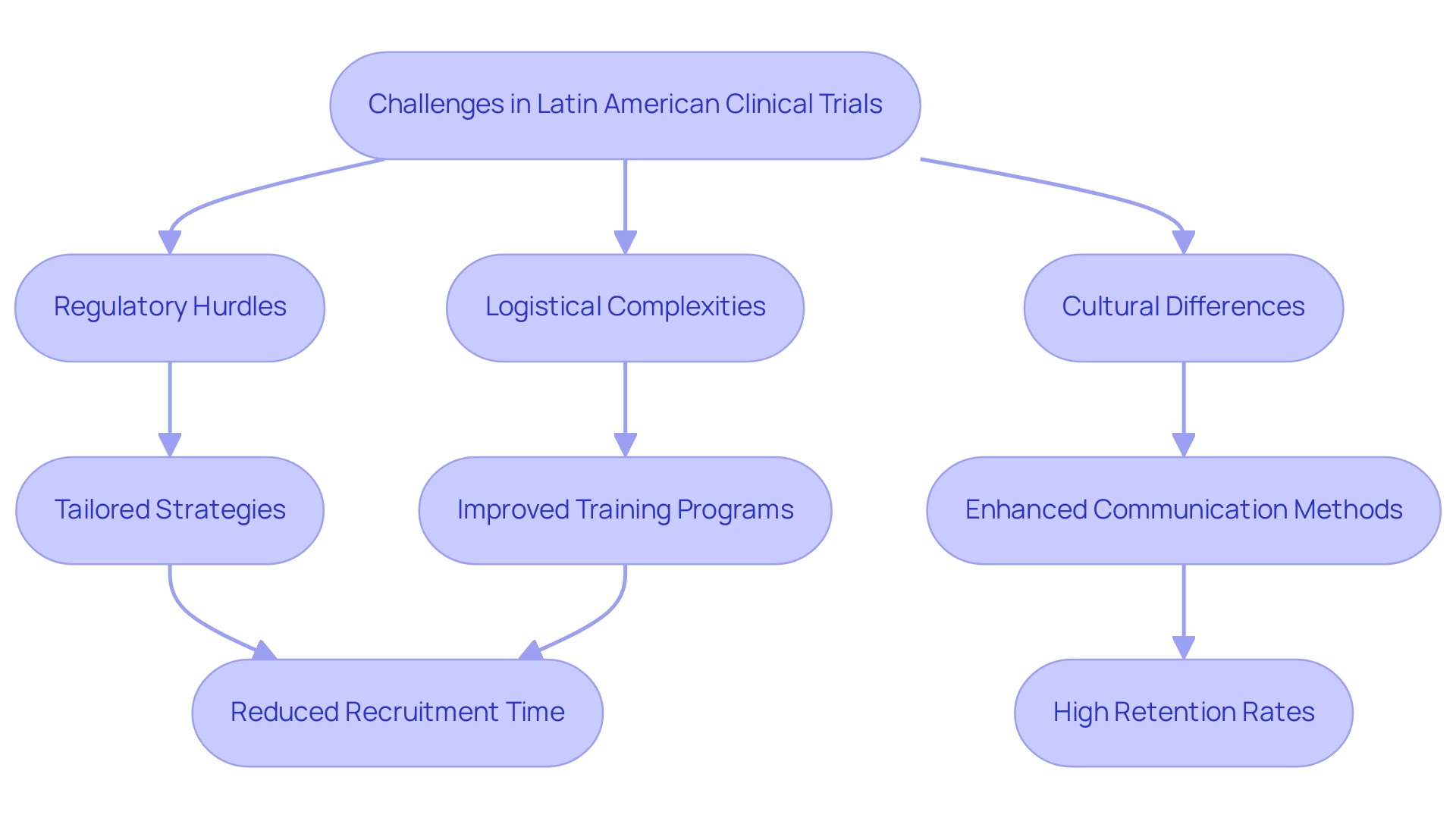
Conducting medical studies in Latin America presents a distinct set of challenges, despite the region's many advantages. Regulatory hurdles, logistical complexities, and cultural differences can significantly impede progress. Navigating the diverse regulatory landscape across countries necessitates tailored strategies to ensure compliance and efficiency.
Moreover, logistical issues such as supply chain management and patient recruitment complicate the execution of studies. However, innovative solutions are emerging to tackle these obstacles. Improved training programs for local investigators are being introduced to ensure they possess the necessary knowledge of both regulatory requirements and best practices in research. Enhanced communication methods between sponsors and local teams foster better collaboration and understanding, which are crucial for successful outcomes.
As Julio Martinez noted in the LATAM Medtech Leaders Podcast, "Comprehending local market dynamics and nurturing connections with local stakeholders is vital for success in research studies."
Statistics reveal that dropout rates in Latin America are significantly lower—approximately one-third of those observed in the U.S. and EU—indicating a strong commitment from participants and a favorable environment for studies. This statistic underscores the potential for successful execution in the region when challenges are effectively managed.
Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, emphasizes the importance of this commitment, asserting, "Our devotion to regulatory excellence and innovation in medtech exploration is essential for managing the intricacies of medical evaluations."
Case studies illustrate the success stories emerging from Latin American trials, particularly those organizations that have embraced these solutions. For instance, bioaccess® demonstrates how connecting innovative Medtech companies with the research environment in Latin America can accelerate the progress of medical devices. Their partnership with Caribbean Health Group aims to establish Barranquilla as a key location for medical research, supported by Colombia's Minister of Health.
By leveraging their extensive expertise and tailored strategies, bioaccess® has adeptly managed the complexities of research studies, resulting in success stories in Latin America trials, achieving over a 50% reduction in recruitment time and 95% retention rates, ultimately facilitating the quicker commercialization of innovative medical technologies.
In conclusion, while conducting medical studies in South America presents significant challenges, proactive strategies and innovative solutions, as discussed by industry leaders, are paving the way for success. This makes the region an increasingly attractive option for medical exploration in 2025.
The Impact of CROs on Success in Clinical Trials
Contract study organizations (CROs) play a pivotal role in promoting success stories in Latin American trials for medical studies. With over 15 years of experience in the Medtech sector, bioaccess® exemplifies how CROs enhance the research process, leveraging their comprehensive services in regulatory compliance, site feasibility, investigator selection, project management, and detailed reporting. This expertise is essential for navigating the complex regulatory environment, ensuring trials are conducted effectively while adhering to international standards.
In 2025, the influence of CROs on trial success is more pronounced than ever. Their ability to offer customized solutions for various study phases—from pilot studies to post-market evaluations—enables Medtech companies to accelerate innovations. For example, bioaccess®'s tailored approach not only expedites research studies but also fosters a collaborative atmosphere that prioritizes the timelines and needs of sponsors.
As highlighted by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during his initial human experimentation experience with bioaccess® in Colombia, this collaboration significantly enhances experimentation efficiency and effectiveness.
Colombia presents competitive advantages for first-in-human studies, including cost efficiency, regulatory speed, high-quality healthcare, effective patient recruitment, and R&D tax incentives. The process for obtaining research study approval in Colombia, encompassing IRB/EC endorsement, INVIMA authorization, and MinCIT import permits, is streamlined by CROs like bioaccess®, who are adept at ensuring compliance with local regulations. Furthermore, bioaccess® facilitates the nationalization of investigational devices, a crucial element for success.
Moreover, success stories in Latin American trials illustrate the transformative impact of CROs on outcomes. Advancements in AI and data management are empowering safety teams to strengthen their data foundations, leading to enhanced collaboration and automation in pharmacovigilance. In 2025, safety teams are anticipated to bolster their data foundations with standardized processes, fostering improved collaboration and automation through AI, ultimately elevating the quality of safety reporting and data management.
As the landscape of research studies evolves, the contributions of CROs like bioaccess® remain indispensable. Their commitment to understanding the local context and regulatory requirements positions them as vital partners in the Medtech sector, facilitating the successful advancement of medical devices that can significantly enhance patient outcomes. Reporting on study status, inventory, and serious and non-serious adverse events further emphasizes the critical role that bioaccess® plays in ensuring comprehensive oversight throughout the research process.
Future Innovations in Latin American Clinical Trials
The future of medical studies in Latin America is on the verge of significant transformation, propelled by innovative technological advancements and evolving regulatory frameworks. Key trends are emerging, particularly the incorporation of artificial intelligence (AI) in study design, which is poised to revolutionize research methodologies. AI's ability to analyze extensive datasets can streamline patient recruitment, refine study protocols, and enhance data analysis, ultimately leading to more efficient and effective studies.
As Peng Lu, chief medical officer of Dutch biotech Pharvaris, observes, 'Homogenizing the use of specific outcomes and outcome measures for clinical studies will support clinical guidelines development and future indirect comparisons among interventions.' This statement underscores the critical importance of standardized outcomes in this dynamic landscape.
Moreover, there is an increasing emphasis on patient-centric approaches, ensuring that studies are crafted with the needs and experiences of participants at the forefront. This shift not only boosts recruitment and retention rates but also enhances the relevance of outcomes to real-world patient experiences. Additionally, the growing focus on diversity in medical studies is vital, as inclusive experiments are essential for developing medical devices that cater to a broad spectrum of populations.
As these innovations unfold, they will significantly enhance the efficiency and effectiveness of medical studies, contributing to success stories in Latin America and establishing South America as a pivotal player in the global research arena. With over 20 years of experience and specialized knowledge, bioaccess® is at the forefront of this evolution, offering expedited medical device research services that connect innovative Medtech companies with the abundant opportunities in South America. Their expertise encompasses Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, ensuring that studies are not only innovative but also relevant to diverse populations.
Bioaccess® prides itself on its adaptability to the unique challenges of each study, providing a tailored approach that steers companies toward successful acquisitions. However, as Max Baumann from Treehill Partners cautions, the biotech market faces fundamental business model challenges amid increasing competition. The region is positioned not only to contribute to local health initiatives but also to play a vital role in advancing global health outcomes.
Key Takeaways from Latin America Trials Success Stories
The success stories arising from medical experiments in Latin America trials unveil several crucial insights that can direct upcoming investigative efforts. Central to these insights is the significance of adaptability in research design, enabling researchers to respond effectively to the dynamic nature of the healthcare environment. For instance, the integration of digital health technologies, such as telemedicine and mobile health applications, has significantly enhanced patient recruitment and monitoring processes.
This technological advancement not only streamlines operations but also enhances the overall quality of medical research, showcasing why Latin America, particularly Barranquilla, is becoming a leading location for trials.
A key example of this is the partnership between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, which aims to position Barranquilla as the most appealing destination for research in Latin America. This partnership, revealed on March 29, 2019, at PROCOLOMBIA's office in Miami, emphasizes the opportunity for local stakeholders to improve the implementation of successful studies by utilizing regional expertise and comprehending specific healthcare requirements.
Furthermore, nurturing robust collaborations among stakeholders—including researchers, healthcare providers, and regulatory entities—is crucial for addressing challenges and optimizing the potential of research initiatives. The MEA medical research market, valued at USD 859.6 million in 2024, is anticipated to expand at a CAGR of 4.1% from 2025 to 2034, signifying a strong opportunity for innovative collaborations. Organizations like bioaccess®, specializing in Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, have achieved over a 50% reduction in recruitment time and maintained a remarkable 95% retention rate in its partnership with GlobalCare Clinical Trials.
This illustrates how flexible approaches, like focused outreach and community involvement, can greatly enhance healthcare outcomes. Additionally, studies contribute favorably to local economies through job creation, economic development, and healthcare enhancement, emphasizing the significance of these initiatives. In summary, the key takeaways from research studies in South America highlight success stories in Latin America trials, underscoring the importance of adaptability, local knowledge, and teamwork, while offering a roadmap for future efforts in this dynamic and evolving environment.
bioaccess® remains dedicated to connecting innovative medtech firms and research opportunities in South America, strengthening its distinct value proposition in this context.
Navigating the Regulatory Landscape for Clinical Trials
Navigating the regulatory environment for research studies in South America presents unique challenges due to the diverse regulations across various nations. However, significant strides have been made in recent years to enhance regulatory frameworks, resulting in more streamlined processes and increased transparency. The anticipated compound annual growth rate (CAGR) for research studies in the region is expected to reach 12.25% from 2025 to 2030, reflecting an increasing interest in conducting experiments in Latin America.
Grasping these changing regulations is crucial for sponsors and contract research organizations (CROs) to ensure adherence and promote the successful implementation of medical studies. Interacting with local regulatory authorities, such as the INVIMA (Colombia's National Food and Drug Surveillance Institute), early in the planning stage can clarify requirements and enhance the effectiveness of logistics. The INVIMA plays a crucial role in medical device oversight and classification as a Level 4 health authority by PAHO/WHO, underscoring its importance in the regulatory landscape.
A case study titled "Regulatory Compliance and Efficiency in Clinical Trials" emphasizes the necessity for stakeholders to understand local regulations established by national health authorities. The outcome of this proactive engagement not only expedites the approval process but also enhances overall operational effectiveness. This sentiment is echoed by Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia.
Moreover, bioaccess provides extensive trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on study status and adverse events
Expert opinions highlight the significance of supply chain management in medical studies, emphasizing its critical role in gaining insights and maintaining operational effectiveness. As one US TPS Business Development Manager noted, "The response was good, and I got what I was looking for as far as the report. Thank you for that," highlighting the value of clear communication and understanding in navigating these complexities.
Additionally, regulatory harmonization efforts are progressing, positioning the region as a more influential participant in global research. By remaining aware of recent regulatory changes and enhancements, stakeholders can strategically position themselves for success in the South American research environment. This proactive approach will lead to success stories in Latin America trials and promote medical innovations more swiftly.
Conclusion: The Future of Medtech Trials in Latin America
The outlook for Medtech studies in South America is exceptionally bright, driven by a robust focus on innovation, collaboration, and patient-centric methodologies. As the area adapts to the evolving landscape of medical research, the insights gained from successful trials in Latin America will serve as a foundation for future initiatives, reinforcing South America's role as a significant contributor to global medical investigations. Notably, the medical technology market in South America was valued at over 44 billion U.S. dollars in 2023, with medical devices comprising more than 80 percent of this total, according to research expert Jennifer Mendoza, underscoring the sector's importance.
Innovative strategies, such as those observed in Chile, where a blend of regulatory efficiency, adherence to international standards, and a diverse patient population has led to enhanced study representativeness, exemplify the success stories in Latin America trials and showcase the region's potential. This multifaceted approach, supported by a robust healthcare system, ethical oversight, and creative patient enrollment methods, has established Chile as a reliable partner for Medtech firms, resulting in high retention rates in research studies.
Furthermore, bioaccess® stands out as the premier CRO in South America, providing comprehensive management services for research initiatives, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies. Their proficiency in regulatory navigation and customized solutions for Medtech startups ensures that stakeholders are well-prepared to tackle the increasing complexities of regulations, including data privacy requirements, which present significant challenges. Bioaccess® employs a tailored approach, adjusting their strategies to the unique needs of each study, thereby enhancing flexibility and responsiveness.
As stakeholders continue to adopt cutting-edge technologies and methodologies, they will unlock the full potential of clinical studies in this dynamic region. The future of Medtech trials in South America transcends mere compliance with regulatory demands; it is about cultivating an environment where innovation flourishes, which can lead to success stories in Latin America trials, ultimately enhancing healthcare outcomes and accelerating advancements in medical devices. Additionally, bioaccess®'s services significantly contribute to job creation and economic growth in the region, reinforcing their role as a catalyst for local development.
While North America is projected to lead the regional market in terms of revenue by 2030, and Asia Pacific is expected to be the fastest-growing market, Latin America remains a critical player in the global Medtech landscape.
Conclusion
The future of Medtech trials in Latin America is exceptionally promising, driven by a robust emphasis on innovation, strategic partnerships, and patient-centered approaches. This region stands ready to leverage insights from successful trials to enhance its contributions to global clinical research. With the medical technology market valued at over 44 billion U.S. dollars in 2023, and medical devices making up a significant portion of this total, the importance of this sector cannot be overstated.
Countries like Chile exemplify the region's potential through their commitment to regulatory efficiency and diverse patient recruitment strategies, leading to enhanced trial outcomes and higher retention rates. These advancements underscore the region's capability to serve as a reliable partner for Medtech companies navigating the complexities of clinical trials.
Bioaccess® emerges as a pivotal player in this landscape, offering comprehensive clinical trial management services tailored to the unique needs of Medtech startups. Their expertise in regulatory navigation and customized strategies positions stakeholders to effectively address the challenges posed by evolving regulations, ensuring the success of clinical trials across the region.
As stakeholders continue to adopt innovative methodologies and technologies, the potential for improved healthcare outcomes and accelerated advancements in medical devices will only grow. Latin America is not merely adapting to the demands of the global clinical research environment; it is establishing itself as a vital contributor to the ongoing evolution of Medtech. The region's commitment to fostering innovation while promoting local economic growth will undoubtedly play a crucial role in shaping the future of healthcare worldwide.
Frequently Asked Questions
What has contributed to the growth of research studies in Latin America?
The region has seen a significant increase in investment for clinical trials, with funding in the Andean Region rising from $3-4 million to over $50 million in the last decade. This growth is particularly notable in Brazil, Argentina, and Mexico.
What role do partnerships play in medical studies in Latin America?
Partnerships, such as the one between bioaccess™ and Caribbean Health Group, are crucial for establishing attractive locations for medical studies, like Barranquilla, as endorsed by Colombia's Minister of Health.
How is research funding distributed in Latin America?
Approximately 69% of research studies are funded by corporations, while universities contribute about 12%, indicating a strong collaboration between academia and industry.
What cultural factors influence patient involvement in research studies in Latin America?
Cultural attitudes towards medical authority can lead patients to consent to participate in studies without fully discussing treatment options or risks with their physicians, highlighting the need to address these nuances for better patient protection.
What is the current situation regarding pediatric research trials in Latin America?
There is a pressing need for more research trials involving pediatric populations, as current participation rates are low. Countries are encouraged to develop incentives for these studies while prioritizing the rights and well-being of vulnerable participants.
What are First-in-Human (FIH) evaluations, and why are they important?
FIH evaluations are essential testing phases for new medical devices and therapies, critical for assessing safety and efficacy and laying the groundwork for subsequent research phases.
What recent trends have been observed in study registrations between high-income nations and Latin America?
Between 2020 and 2023, high-income nations experienced a 12% decline in study registrations, while Latin America is emerging as an alternative center for medical studies due to its diverse patient populations and favorable regulatory environments.
What legislative advancements have been made in Brazil regarding clinical research?
The endorsement of Law 14.874/24 in May 2024 aims to simplify the assessment procedures for studies, reducing bureaucratic obstacles and enhancing predictability for clinical research activities.
What advantages does Latin America offer for conducting medical trials?
Latin America provides lower operational costs, access to diverse patient demographics, and a streamlined regulatory environment, making it an appealing choice for Medtech research studies.
How does bioaccess® support Medtech firms in conducting studies in South America?
Bioaccess® offers a personalized approach to managing studies, including feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting, connecting innovative Medtech firms with research opportunities.

