Introduction
In the complex and highly regulated field of drug development, the significance of conducting adequate and well-controlled studies cannot be overstated. These studies serve as the backbone for establishing the efficacy and safety of new pharmaceuticals, ensuring that the data collected is both reliable and valid.
Central to this process is the adherence to rigorous standards set forth by regulatory bodies, such as the FDA, which mandates the inclusion of control groups and the implementation of sound methodologies to mitigate bias.
As the landscape of clinical trials evolves, particularly in regions like Latin America, the integration of advanced technologies and adaptive trial designs is reshaping how researchers approach study execution.
This article delves into the critical components, regulatory frameworks, and emerging trends that define the future of clinical trials, highlighting the challenges faced and the innovative solutions being adopted to enhance trial outcomes and drive medical advancements.
Defining Adequate and Well-Controlled Studies in Drug Development
In the realm of drug development, 'adequate and well-controlled investigations' are critical for establishing a reliable foundation for assessing both the efficacy and safety of new pharmaceuticals. These investigations must incorporate a control group, which can be either a placebo or an active comparator, as specified in 21 cfr 314.126. This design is essential for enabling valid comparisons of treatment effects.
For example, the overall response rate (ORR) of atezolizumab combined with bevacizumab in extra pancreatic NET was reported to be 15%, highlighting the significance of strong research designs in producing meaningful outcomes. To be regarded as adequate, an investigation must be sufficiently powered and conducted over a duration that allows for the collection of statistically meaningful results. Furthermore, well-controlled research is structured to minimize bias, thereby enhancing the integrity of the data.
Our extensive healthcare research management services assist in:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project configuration
- Project oversight
All of which are essential for ensuring adherence to regulations and promoting the success of medical research. We also offer comprehensive reporting on study status, inventory, and both serious and non-serious adverse events, along with help regarding import permits for investigational devices, ensuring a seamless transition through approval processes. The incorporation of emerging technologies, such as AI and machine learning, is also transforming medical studies, contributing to their efficiency and effectiveness.
This definition is crucial in shaping regulatory expectations for medical studies, ensuring that the data submitted during the drug approval process is not only robust but also credible. As Yuan-Sheng Zang observed, 'These authors contributed equally,' highlighting the collaborative efforts necessary in medical research. The emphasis on control groups is particularly significant, as they provide a benchmark against which the treatment's effects can be measured, reinforcing the overall validity of the results.
Furthermore, the influence of Medtech research initiatives goes beyond prompt outcomes, promoting job creation, economic development, and enhancements in healthcare, while also encouraging international cooperation, which is crucial for progressing medical research in Latin America and beyond.
The Regulatory Importance of 21 CFR 314.126 in Clinical Trials
The execution of research studies is largely governed by 21 CFR 314.126, which establishes strict standards vital for assessing the safety and effectiveness of new medications. The FDA heavily relies on compliance with this regulation when assessing new drug applications, mandating that studies be adequately designed and well-controlled. This requirement protects participants engaged in research studies and guarantees the validity and relevance of the data gathered.
Non-compliance with 21 CFR 314.126 can result in significant repercussions, such as:
- Delayed approvals
- Increased scrutiny from oversight bodies
- Outright rejection of drug applications
This highlights the regulation's critical importance in the drug development lifecycle. For Medtech companies navigating the complexities of clinical trials in Latin America, the expertise of professionals like Katherine Ruiz in compliance matters becomes crucial. Furthermore, the FDA's guideline under 21 CFR 314.600 permits drug approval based on animal research when human efficacy trials are not ethical or practical, showcasing flexibility in oversight.
The FDA emphasizes that the rigor of data collection is paramount, often outweighing the size of studies, particularly in cases with external controls. This trend reflects a growing adaptability in the approval process, especially for orphan products addressing unmet medical needs. Between 2000 and 2019, the FDA approved 45 products relying on external controls, primarily for rare diseases, indicating a significant shift in regulatory practices.
As Russell Katz, M.D. from the FDA states, 'the only studies that can be unambiguously interpreted to provide evidence of effectiveness... are those in which a difference between active treatment and the control group is detected.' Adhering to 21 CFR 314.126 not only supports the integrity of research studies but also significantly impacts drug approval schedules and overall compliance with FDA regulations.
Comprehensive health research management services, including:
- Feasibility assessments
- Site selection
- Project setup
- Compliance reviews
- Import permits
- Project management
- Reporting
are essential in addressing the challenges faced by Medtech companies in Latin America, ensuring a streamlined process from innovation to execution.
Key Components of Adequate and Well-Controlled Studies
To ensure the integrity and reliability of clinical trials, adequate and well-controlled research must encompass several fundamental components:
-
Randomization: Participants should be randomly assigned to treatment and control groups to mitigate selection bias, ensuring that external factors do not influence the outcomes. Research has indicated significant variations in practices among Clinical Trial Units (CTUs) regarding blinding, influenced by factors such as design, types of interventions, and funding availability.
For instance, the variation in randomization practices highlights the importance of utilizing standardized methods to achieve balance in group assignment.
-
Blinding: Implementing double-blind designs—where neither participants nor researchers are aware of group assignments—plays a crucial role in minimizing bias in outcome assessment.
Research by MacLeod et al. indicates that research lacking randomization or blinding tends to report substantially higher effect sizes, underscoring the necessity of these methodologies.
-
Sample Size Calculation: Accurate sample size determination using statistical methods is essential to ensure the research possesses sufficient power to detect meaningful effects. This calculation is essential in drug research, as insufficient sample sizes can lead to inconclusive results, ultimately affecting the validity of the findings.
-
Clear Endpoints: Defining medical endpoints that are directly relevant to patient outcomes is vital for the precise assessment of efficacy and safety. These endpoints should be unambiguous and reflective of the treatment's intended benefits.
Furthermore, several approaches for executing randomization, including coin flips, dice throws, and random number generators, have been recorded in case analyses, offering researchers practical tools to improve reproducibility and transparency in research designs.
Moreover, extensive research project management services, including feasibility assessments, site selection, compliance evaluations, project initiation, and management, cover essential functions such as review and feedback on research documents and detailed reporting of project status, inventory, and adverse events. By adhering to these key components and leveraging these services, researchers can generate credible results that meet regulatory standards while contributing valuable insights to the clinical field.
Challenges in Conducting Adequate and Well-Controlled Studies
Conducting adequate and well-controlled research presents numerous challenges that can significantly impact trial outcomes. Key issues include:
-
Recruitment Issues: Enrolling qualified participants remains a persistent hurdle, particularly for research with stringent demographic requirements or specific medical conditions.
Notably, as evidenced by recent trends, the person-to-person recruitment method is employed by 93% of studies; however, it has not shown a direct correlation with accrual percentages, highlighting a critical gap in recruitment effectiveness. This challenge is further compounded by the competitive nature of clinical studies, where medical device startups often face significant regulatory hurdles and financial constraints.
-
Retention of Participants: Maintaining participant engagement throughout the study is critical for ensuring data integrity and achieving completion. Successful research, such as that carried out by GlobalCare Clinical Trials in partnership with bioaccess™, has shown that in-person recruitment methods can result in a 100% completion rate among screened subjects. This underscores the importance of effective retention strategies, as evidenced by GCCT's reported increase in subject retention rates to over 95% after partnering with bioaccess™ to enhance its ambulatory services in Colombia.
The trial setup services provided included comprehensive project management and ongoing support, which were pivotal in achieving these results.
-
Data Management: Precise data gathering and management are essential, as mistakes can compromise research outcomes and threaten compliance with regulations.
Expert opinions stress that a gap exists in understanding patient perspectives within the recruitment process. A representative from a pharmaceutical company noted,
One of the root causes of recruitment failure is that almost nobody except the sites actually understands the patients.
This emphasizes the necessity for closer cooperation between sponsors and participants to improve the design.
Integrating this perspective into the recruitment process can significantly improve outcomes.
-
Regulatory Compliance: Navigating the complex landscape of regulations, such as 21 CFR 314.126, and ensuring continuous compliance can be resource-intensive, requiring meticulous attention to detail.
The services provided in preliminary setup, including review of research documents for compliance with 21 CFR 314.126, obtaining import permits, and reporting on project status and adverse events, are essential in addressing these regulatory challenges. Tackling these challenges during the planning phase is vital for the successful implementation of research trials. Future research should consider employing mixed-methods approaches and advanced analytics to refine recruitment strategies, as recommended in recent case analyses.
By proactively identifying and addressing these issues, researchers can improve participant recruitment and retention, ultimately leading to more robust and dependable results.
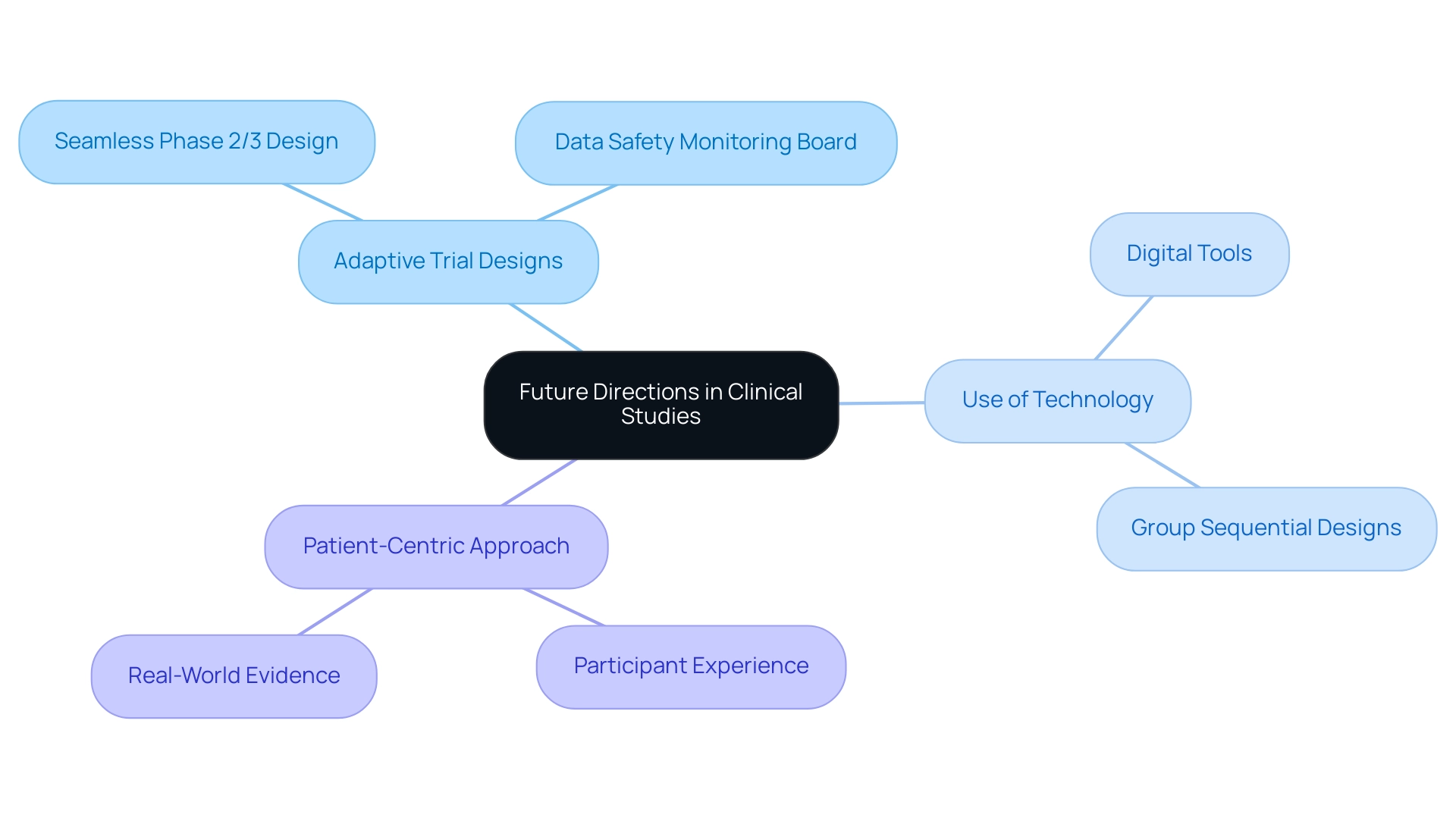
Future Directions in Adequate and Well-Controlled Studies
The terrain of clinical experiments is constantly changing, affected by various trends that are poised to define the future of sufficient and well-regulated research. One prominent trend is Adaptive Trial Designs, which facilitate modifications to study protocols based on interim results, significantly enhancing flexibility and efficiency. These designs are particularly applicable in situations where early evidence suggests that a treatment may be more effective than initially anticipated or when safety concerns arise.
For instance, the seamless Phase 2/3 design exemplifies how researchers can combine learning and confirmatory phases into a single study, allowing for an immediate transition from Phase 2 to Phase 3 based on the most efficacious dose observed. This approach not only optimizes resource allocation but also accelerates the drug development process, a critical aspect for Medtech companies aiming to innovate in challenging environments like Latin America.
However, safety considerations in adaptive designs must account for the potential risks to participants. It is suggested that a data safety monitoring board be created to direct study modifications, ensuring that participant safety stays a top priority throughout the research.
The Use of Technology in medical studies is also increasing, with digital tools like electronic data capture and telemedicine playing an essential role in streamlining processes and enhancing participant engagement. Notably, group sequential designs have been utilized in 18.8% of COVID-19 studies, showcasing the practical application of adaptive strategies during urgent health crises. Furthermore, the integration of technology has proven to enhance testing efficiency, making it an indispensable component of modern clinical research.
Another significant trend is the Patient-Centric Approach, which emphasizes the experience and involvement of participants in research design. By concentrating on patient needs, researchers can enhance recruitment and retention rates, ultimately resulting in more successful study outcomes. Furthermore, the integration of Real-World Evidence into research frameworks provides a supplementary viewpoint, enhancing conventional results and delivering greater understanding of drug effectiveness.
As the field progresses, it is essential for researchers, particularly those involved with Medtech advancements in Latin America, to stay adaptable and receptive to these revolutionary changes. Expert insights highlight the significance of adjusting to these trends, as they not only guarantee adherence to standards but also improve overall study outcomes. The partnership between Greenlight Guru and bioaccess™ is a testament to the innovative methods being employed to expedite clinical studies in Latin America, as highlighted by PAVmed's First In-Human Study in Colombia.
This collaboration can lead to substantial impacts on local economies through job creation and improved healthcare outcomes. Moreover, tackling the specific challenges encountered by US Medtech companies, such as regulatory hurdles and language barriers, is essential for successful execution. Comprehensive services, including feasibility studies, site selection, compliance reviews, trial setup, and project management, are essential to navigate these challenges effectively.
Conclusion
The future of drug development hinges on the successful execution of adequate and well-controlled studies, which are fundamental to validating the efficacy and safety of new pharmaceuticals. As highlighted throughout the article, adherence to the stringent regulatory standards set forth by the FDA, particularly 21 CFR 314.126, is critical for ensuring the integrity of clinical trials. These regulations not only serve to protect participants but also to reinforce the credibility of the data that underpins drug approval processes.
The challenges faced in conducting these studies, such as:
- Recruitment and retention issues
- Data management
- Regulatory compliance
require innovative solutions and strategic planning. By leveraging advanced technologies and adaptive trial designs, researchers can enhance trial efficiency and participant engagement, thereby improving overall outcomes. The emphasis on a patient-centric approach and the integration of real-world evidence further enriches the data landscape, providing a holistic understanding of treatment effectiveness.
As the clinical trial landscape continues to evolve, particularly in regions like Latin America, the collaboration between stakeholders and the application of comprehensive clinical trial management services will be vital. These efforts not only facilitate compliance with regulatory frameworks but also drive forward the innovation necessary for addressing unmet medical needs. Ultimately, the commitment to rigorous study design and execution will shape the future of medical advancements, paving the way for safer and more effective therapies for patients worldwide.
Frequently Asked Questions
What are 'adequate and well-controlled investigations' in drug development?
They are essential studies that establish a reliable foundation for assessing the efficacy and safety of new pharmaceuticals, incorporating a control group for valid comparisons of treatment effects.
What is the significance of having a control group in clinical investigations?
A control group, which can be a placebo or an active comparator, allows for valid comparisons of treatment effects, enhancing the integrity and validity of the research outcomes.
What requirements must an investigation meet to be considered adequate?
An investigation must be sufficiently powered, conducted over a duration that allows for the collection of statistically meaningful results, and structured to minimize bias.
What services are offered to assist in healthcare research management?
Services include feasibility assessments, site selection, compliance evaluations, project configuration, and project oversight, all essential for adhering to regulations and promoting successful medical research.
How does the FDA regulate drug development studies?
The FDA relies on compliance with 21 CFR 314.126, which sets strict standards for the design and control of studies to ensure the safety and effectiveness of new medications.
What are the consequences of non-compliance with 21 CFR 314.126?
Non-compliance can lead to delayed approvals, increased scrutiny from oversight bodies, and outright rejection of drug applications.
How does the FDA's guideline under 21 CFR 314.600 provide flexibility in drug approval?
It allows for drug approval based on animal research when human efficacy trials are not ethical or practical, demonstrating adaptability in oversight.
What trend has been observed regarding the size of studies and data collection rigor?
The FDA emphasizes that the rigor of data collection is more important than the size of studies, especially for cases with external controls, reflecting adaptability in the approval process.
What role do comprehensive health research management services play for Medtech companies?
They address challenges faced by Medtech companies in clinical trials, ensuring a streamlined process from innovation to execution, including services like project management and reporting.
What impact does adherence to 21 CFR 314.126 have on drug approval schedules?
Adhering to this regulation supports the integrity of research studies and significantly impacts drug approval schedules and overall compliance with FDA regulations.




