Overview
The article focuses on the advancements and potential of clinical research infrastructure in Latin America, highlighting its benefits, key players, and emerging trends. It emphasizes that countries like Colombia, Brazil, and Mexico are becoming attractive sites for clinical trials due to cost-effectiveness, diverse patient populations, and supportive regulatory frameworks, which collectively enhance the region's capability to conduct high-quality medical research.
Introduction
As the global landscape of clinical research evolves, Latin America emerges as a vibrant hub for innovation and opportunity. With countries like Brazil, Mexico, and Colombia at the forefront, the region boasts diverse patient populations and robust healthcare systems that make it an attractive destination for clinical trials.
This article delves into the remarkable transformation of clinical research in Latin America, exploring the advantages of conducting trials in this region, the rise of decentralized clinical trials, and the pivotal role of Contract Research Organizations (CROs) in navigating complex regulatory landscapes.
Additionally, it highlights the challenges faced in recruitment and operational hurdles, while projecting a future filled with growth and technological advancements that promise to reshape the industry.
Overview of Clinical Research in Latin America
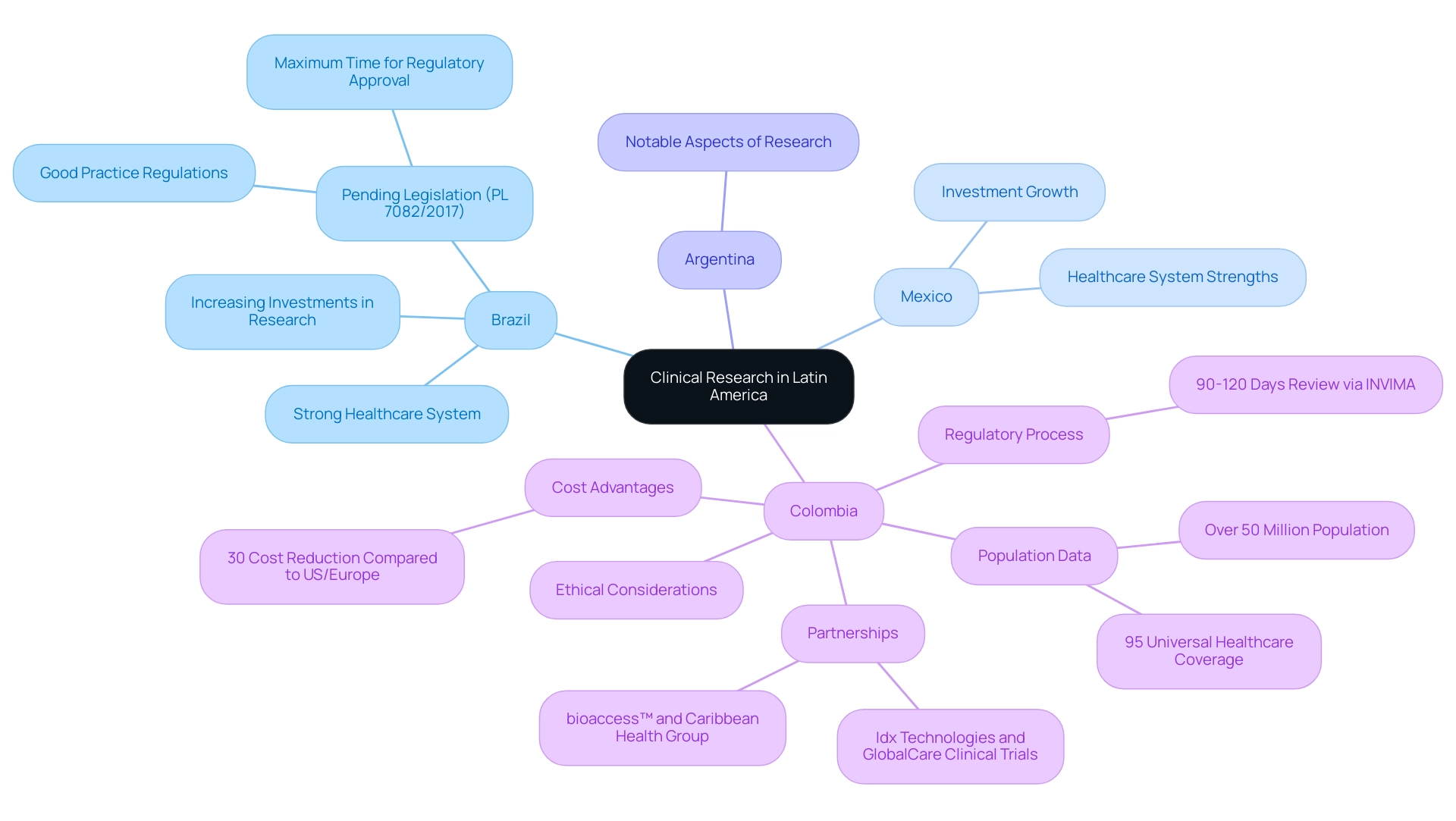
The Latin America clinical research infrastructure has experienced significant development, with numerous countries positioning themselves as key sites for conducting medical evaluations. The region is characterized by a diverse patient population, which is essential for evaluating the efficacy of treatments across various demographics. Countries like Brazil, Mexico, and Argentina are leading this transformation of the Latin America clinical research infrastructure, each boasting strong healthcare systems and increasing investments in research infrastructure.
Remarkably, Colombia stands out as a premier location for first-in-human (FIH) studies, providing substantial competitive advantages. The nation boasts cost reductions of over 30% compared to tests in the United States or Western Europe, a swift regulatory review process of 90-120 days via INVIMA, and a healthcare system ranked among the best worldwide by the World Health Organization. Furthermore, with a population exceeding 50 million and 95% covered by universal healthcare, patient recruitment is robust.
Hospitals in Colombia are only permitted to carry out medical studies with pharmaceutical drugs after they have undergone a stringent ICH/GCP certification process, ensuring high-quality standards. The recent partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as the most appealing location for medical studies, leveraging the Latin America clinical research infrastructure, with strong backing from Colombia's Minister of Health, which is essential for creating a supportive atmosphere for research. Additionally, global partnerships, such as those with Idx Technologies and GlobalCare Clinical Trials, are enhancing services and efficiency in the region, achieving reductions in recruitment time and high retention rates.
These developments not only emphasize the region's potential but also underscore the importance of addressing ethical considerations and navigating cultural nuances in ongoing and future assessments.
Advantages of Conducting Clinical Trials in Latin America
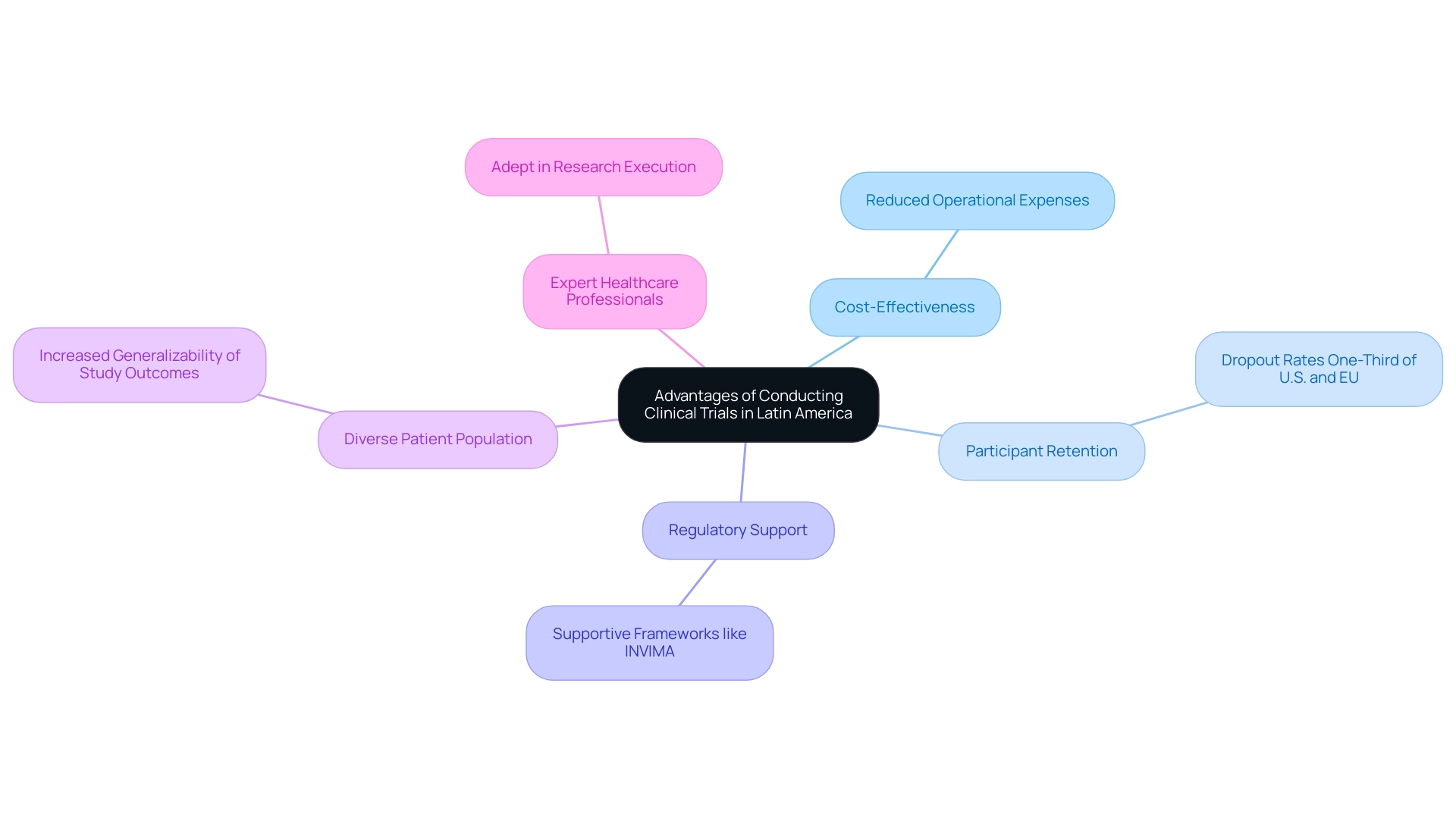
Carrying out medical studies within the Latin America clinical research infrastructure offers a striking range of benefits, especially recognized for their reduced operational expenses in comparison to North regions and Europe. This cost-effectiveness is significant, as dropout rates in Latin regions are impressively one-third of those observed in the U.S. and EU, showcasing the area's commitment to participant retention. Moreover, the Latin America clinical research infrastructure includes a sizable and varied patient population, which increases the generalizability of study outcomes and permits the inclusion of diverse demographic factors that are essential in medical investigations.
Numerous nations in this region have also established supportive regulatory frameworks, including INVIMA, Colombia's National Food and Drug Surveillance Institute, which is categorized as a Level 4 health authority by PAHO/WHO, thereby enhancing the Latin America clinical research infrastructure through quicker approvals and streamlined processes for study initiation. The robust presence of adept healthcare experts familiar with research enhances the effectiveness of execution in the Latin America clinical research infrastructure. bioaccess® provides extensive clinical study management services, specializing in:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
ensuring a customized approach tailored to the needs of clients.
With over 20 years of experience in the Medtech industry, bioaccess® has the expertise and flexibility necessary to adapt to the unique requirements of each study. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, attests to the expertise of bioaccess® during their initial human testing in Colombia, highlighting the value of their services. Additionally, a recent case study titled 'U.S. Market Outlook' indicates that the growth outlook for the U.S. market has been raised, reflecting higher recent growth and an expected increase in patient use of higher-value therapies. This comparative viewpoint emphasizes the benefits of conducting experiments within the Latin America clinical research infrastructure, where the conditions are becoming more advantageous. As Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, mentions, he is personally and passionately dedicated to assisting biotech leaders in the planning and execution of phase I-IV studies across different areas, including Latin.
This emphasizes the knowledge accessible to sponsors, ultimately making the Latin America clinical research infrastructure a more appealing choice for research operations.
Emerging Trends: Decentralized Clinical Trials in Latin America
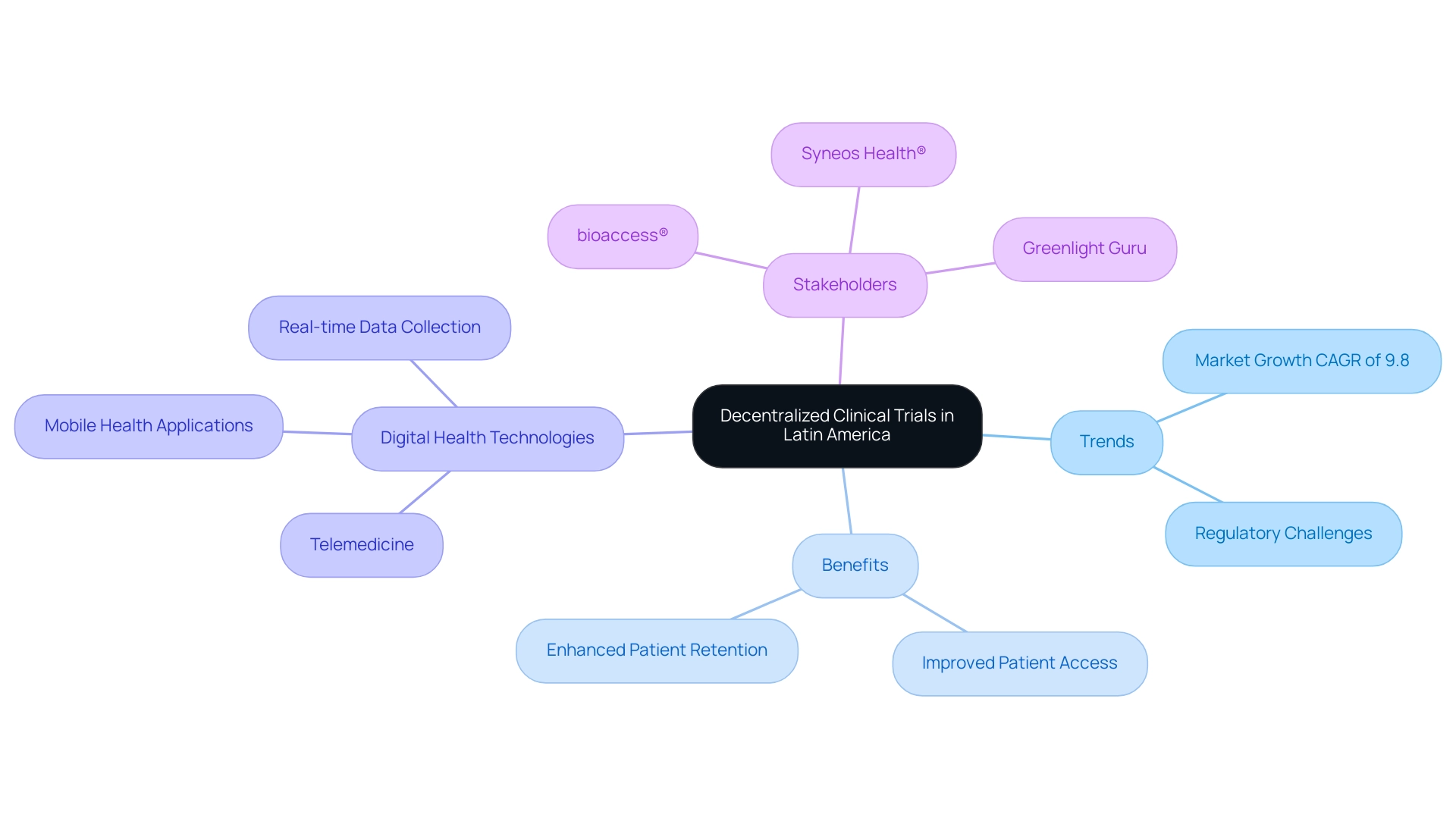
Decentralized medical studies (DCTs) are transforming the Latin America clinical research infrastructure by offering a flexible structure that improves patient access while tackling the distinct obstacles encountered by Medtech firms in the region, including regulatory challenges and language barriers. This innovative model significantly enhances patient retention and effectively reduces geographical barriers, making it particularly relevant for organizations like bioaccess®, a leading contract research organization facilitating medical device clinical studies. The integration of digital health technologies, including telemedicine and mobile health applications, further supports the seamless implementation of DCTs, enabling real-time data collection and enhancement of study processes.
As the decentralized research model continues to gain traction, it is crucial for researchers to adapt their methodologies to fully harness its advantages. Projections suggest that the decentralized research market will expand at a CAGR of 9.8% from 2025 to 2031, emphasizing the urgent need for stakeholders to remain informed. As indicated by Syneos Health®, the creation of a decentralized research site network is essential for enhancing DCT adoption, guaranteeing the provision of high-quality studies backed by cutting-edge digital health technology solutions.
Moreover, the partnership between Greenlight Guru and bioaccess™ illustrates how collaborations can expedite Medtech advancements and studies within the Latin America clinical research infrastructure, highlighted by PAVmed's first-in-human research in Colombia. To bridge the gaps in medical research and innovation, it is essential for stakeholders to adopt a solution-driven approach, keeping abreast of these developments to maximize opportunities in this evolving landscape.
Infrastructure and Resources for Clinical Trials: The Role of CROs
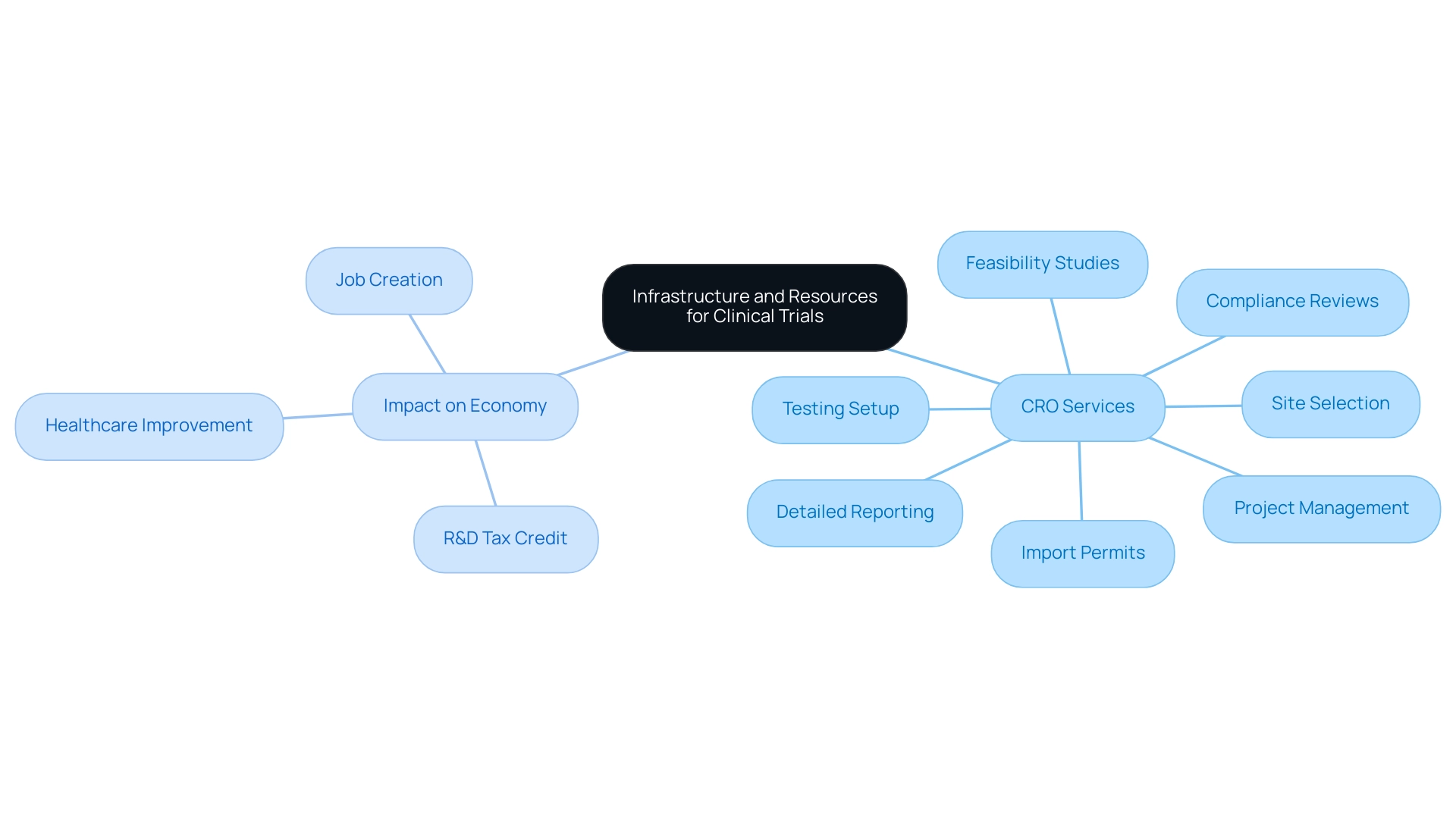
Contract Research Organizations (CROs) such as bioaccess® are essential to the Latin America clinical research infrastructure, offering a comprehensive suite of services that expedite medical device clinical studies. These services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Testing setup
- Import permits
- Project management
- Detailed reporting
By collaborating with specialized CROs, researchers can leverage local insights that are crucial for navigating the complexities of diverse study environments.
Listening to regional experts significantly enhances trial outcomes, as emphasized by Julio G. Martinez-Clark, CEO of bioaccess, who notes that 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.' Furthermore, CROs play a pivotal role in fostering economic growth, job creation, and healthcare improvement, especially with the new Colombian R&D tax credit that allows small and midsize companies to claim a 50% tax credit for their investments in R&D and innovation projects. This encourages partnerships with CROs, promoting international collaboration and innovation in medtech, and ultimately improving the quality and efficiency of studies across the Latin America clinical research infrastructure.
For any queries or concerns regarding the processing of information, clients can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®') via email at info@bioaccessla.com. We are dedicated to addressing all concerns in alignment with applicable law, ensuring the protection of client data throughout the investigation process.
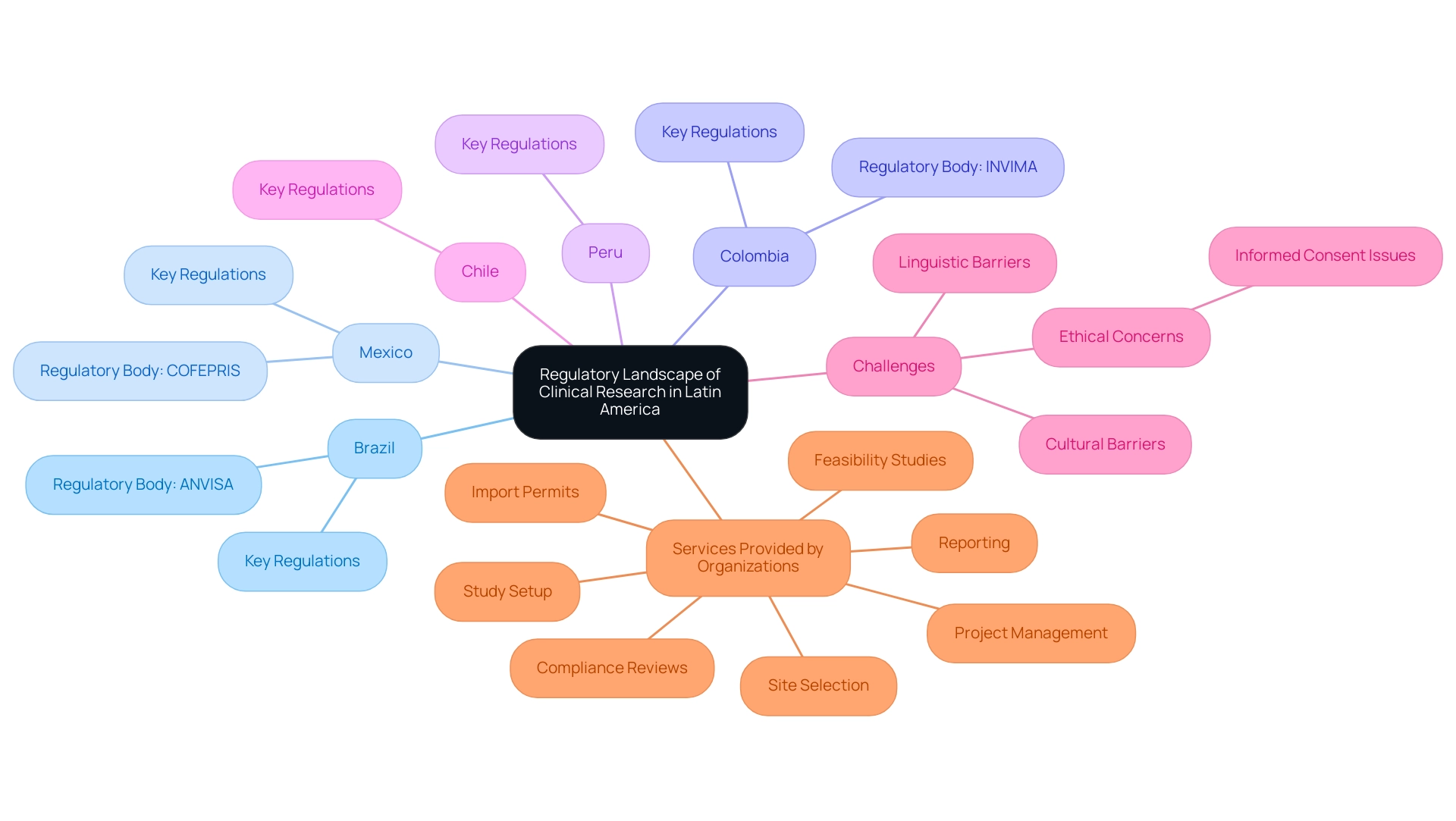
Navigating the Regulatory Landscape of Clinical Research
Navigating the regulatory environment of medical research presents unique challenges in the Latin America clinical research infrastructure due to the diverse regulations that differ across nations. Each nation, such as Brazil with its ANVISA and Mexico with its COFEPRIS, has its own set of laws and ethical guidelines that researchers must thoroughly understand to ensure compliance. Engaging with these agencies early in the study planning phase is crucial for avoiding delays in approvals and ensuring adherence to ethical standards.
Furthermore, as outsourced research trials increase in Peru, Colombia, and Chile, addressing linguistic, cultural, and socio-economic barriers becomes essential for informed consent. In Latin regions, it is noteworthy that patients may sometimes consent to engage in research studies without fully discussing treatment options or risks with their physician, raising significant ethical concerns. Regulatory authorities emphasize the importance of transparency and adherence in research to protect participants and maintain integrity within the industry.
Additionally, language differences pose challenges in informed consent processes, highlighting the need for effective communication strategies. As the pharmaceutical sector keeps expanding in Latin America, it is essential to remain aware of changing regulatory frameworks and compliance strategies to ensure ethical and successful studies within the Latin America clinical research infrastructure. Organizations like bioaccess® provide extensive management services for research studies, including:
- feasibility studies
- site selection
- compliance reviews
- study setup
- import permits
- project management
- reporting
positioning themselves as leaders in facilitating medical device research in this diverse region.
Moreover, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential as it oversees medical device regulation and classification, ensuring adherence to local standards and practices.
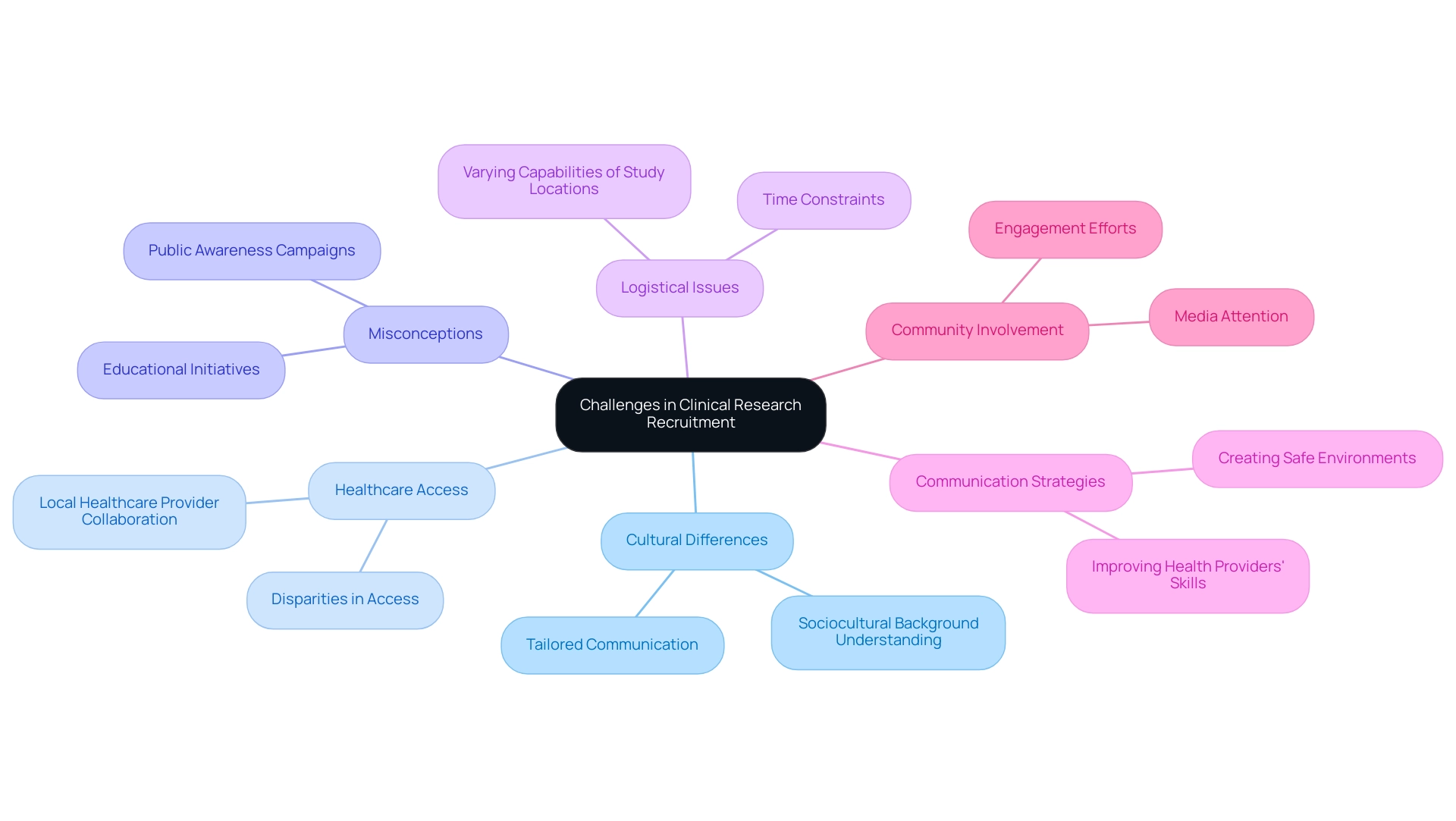
Challenges in Clinical Research: Recruitment and Operational Hurdles
Carrying out medical studies within the Latin America clinical research infrastructure poses distinct obstacles that can obstruct participant recruitment, as emphasized in several Clinical Leader articles examining the environment of medical experiments in the area. Cultural differences, disparities in healthcare access, and pervasive misconceptions about clinical trials often complicate the recruitment process. Logistical issues, such as the varying capabilities of study locations, can significantly affect timelines.
Vyjeyanthi S Periyakoil, MD, emphasizes that understanding participants' sociocultural background is central because it enables researchers to communicate effectively with potential participants. This insight underscores the importance of tailored communication strategies in overcoming recruitment barriers. Furthermore, operational hurdles, such as time constraints—identified as a limiting factor in the enrollment of Hispanic-Latino individuals due to caregiver assistance—highlight the necessity for proactive engagement.
Improving health providers' communication skills is vital, as it enhances participant recruitment by fostering trust and understanding. The case study titled 'Challenges in Diversifying Clinical Trial Subjects' illustrates the ongoing challenges in ensuring that practices reflect the needs of underrepresented groups, emphasizing that without proper changes, the inclusion of diverse subjects does not guarantee improved medical knowledge or treatment outcomes. To tackle these challenges, researchers should emphasize community involvement efforts and invest in educational initiatives that explain the advantages of participating in studies within the Latin America clinical research infrastructure.
Establishing robust collaborations with local healthcare providers not only improves recruitment efforts but also simplifies operational processes, ultimately resulting in more successful medical study outcomes. Furthermore, favorable media attention on medical studies, as demonstrated in Clinical Leader's reporting, can greatly influence local economies by encouraging job creation, boosting economic development, and enhancing healthcare results through heightened involvement in studies.
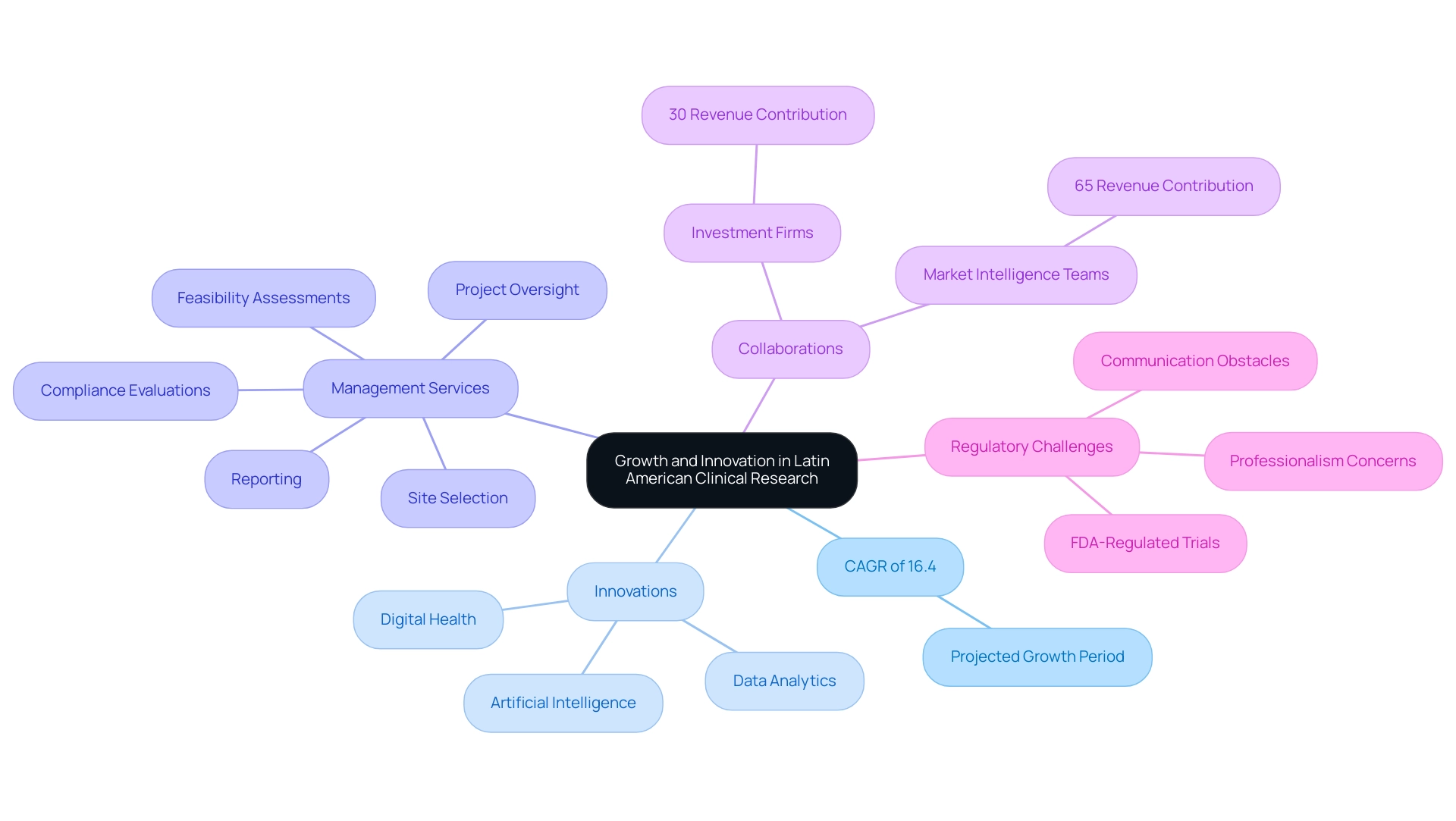
Future Perspectives: Growth and Innovation in Latin American Clinical Research
The Latin America clinical research infrastructure is on the verge of substantial change in medical investigation, propelled by an anticipated compound annual growth rate (CAGR) of 16.4% from 2025 to 2030. Innovations in digital health, artificial intelligence, and data analytics are set to transform research processes, improving efficiency and emphasizing patient-focused methods. Comprehensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
- Review and feedback on study documents
are essential as the Latin America clinical research infrastructure continues to improve.
As pointed out by industry specialists, including Nuray Kenzhebek, a marketing director focused on pharma and biotech, the Latin America clinical research infrastructure's expanding role in FDA-regulated trials underscores a broader trend: the globalization of medical studies, which stresses the need for cooperation among academia, industry stakeholders, and regulatory organizations like INVIMA, Colombia's national food and drug surveillance institute. This authority oversees medical devices and is classified as a Level 4 health authority by PAHO/WHO. The economic impact of Medtech research studies is profound, contributing to job creation, economic growth, healthcare improvement, and fostering international collaboration.
Roughly 30% of revenue in the medical sector originates from collaborations with investment companies, while 65% is produced by competitive and market intelligence teams, highlighting the financial dynamics involved. Moreover, insights from the U.S. market outlook reveal a raised growth forecast, serving as a parallel for anticipated advancements in Latin regions. Tackling the challenges presented by regulatory changes, including those emphasized by recent cannabis seed confiscations, along with communication obstacles and professionalism concerns, will be essential for operational activities.
By harnessing these advancements and tackling such hurdles, the Latin America clinical research infrastructure is not only set to thrive but also to lead in identifying future growth areas, particularly as the integration of AI and data analytics becomes more prevalent.
Conclusion
The evolution of clinical research in Latin America presents a landscape rich with opportunity, driven by diverse patient populations, cost-effective trials, and innovative methodologies. Countries like Colombia, Brazil, and Mexico are leading this transformation, establishing themselves as prime locations for clinical trials due to their robust healthcare systems and supportive regulatory frameworks. The increasing adoption of decentralized clinical trials is further enhancing patient access and retention, showcasing the region's adaptability to modern research needs.
However, the journey is not without challenges. Recruitment hurdles stemming from cultural differences and logistical complexities can impede progress, necessitating tailored communication strategies and community engagement to foster trust and understanding. The role of Contract Research Organizations (CROs) has become pivotal in navigating these obstacles, providing essential services that streamline trial execution and ensure compliance with local regulations.
Looking ahead, the future of clinical research in Latin America is promising, with a projected growth rate that highlights the region's expanding role in global clinical trials. As innovations in digital health and data analytics take center stage, stakeholders must remain proactive in addressing regulatory challenges and enhancing operational efficiencies. By harnessing these advancements and fostering collaboration among industry players, Latin America is poised not only to thrive in clinical research but also to set benchmarks for excellence in the field. The region's commitment to innovation and improvement will undoubtedly shape the future of clinical trials, making it an exciting time for both researchers and participants alike.
Frequently Asked Questions
What recent developments have occurred in the Latin America clinical research infrastructure?
The Latin America clinical research infrastructure has significantly developed, with countries like Brazil, Mexico, and Argentina emerging as key sites for medical evaluations, supported by strong healthcare systems and increased investments in research.
Why is Colombia considered a premier location for first-in-human (FIH) studies?
Colombia is recognized for FIH studies due to cost reductions exceeding 30% compared to the U.S. and Western Europe, a swift regulatory review process of 90-120 days via INVIMA, and a highly ranked healthcare system, with over 50 million people and 95% covered by universal healthcare.
What quality standards must hospitals in Colombia meet to conduct medical studies?
Hospitals in Colombia must undergo a stringent ICH/GCP certification process before conducting studies with pharmaceutical drugs, ensuring high-quality standards.
What partnerships are enhancing the clinical research landscape in Colombia?
The partnership between bioaccess™ and Caribbean Health Group aims to make Barranquilla a prime location for medical studies, supported by Colombia's Minister of Health. Additionally, global partnerships with Idx Technologies and GlobalCare Clinical Trials improve services and efficiency in the region.
What are the benefits of conducting medical studies in Latin America compared to North America and Europe?
Latin America offers reduced operational expenses and lower dropout rates, which are one-third of those in the U.S. and EU. The diverse patient population enhances the generalizability of study outcomes.
How does the regulatory framework in Latin America support clinical research?
Countries like Colombia have established supportive regulatory frameworks, such as INVIMA, which streamline processes for study initiation and enhance the efficiency of approvals.
What types of clinical study management services does bioaccess® provide?
bioaccess® specializes in a range of services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, tailored to client needs.
What expertise does bioaccess® bring to clinical research?
With over 20 years of experience in the Medtech industry, bioaccess® offers the expertise and flexibility necessary to adapt to the unique requirements of each study.
What is the outlook for the U.S. market in relation to clinical research in Latin America?
The growth outlook for the U.S. market has been raised, reflecting higher recent growth and an expected increase in patient use of higher-value therapies, highlighting the advantages of conducting research in Latin America.
What is the overall appeal of the Latin America clinical research infrastructure for sponsors?
The Latin America clinical research infrastructure is appealing due to its cost-effectiveness, diverse patient demographics, supportive regulatory environment, and the availability of knowledgeable healthcare experts, making it a favorable choice for research operations.

