Introduction
In the realm of medical device development, navigating the regulatory landscape is a critical undertaking that can significantly impact a product's market success. The Premarket Approval (PMA) process, established by the FDA, serves as a rigorous pathway for high-risk devices, demanding comprehensive clinical data and extensive documentation to validate safety and effectiveness before market entry.
As manufacturers grapple with increasing wait times and the complexities of compliance, particularly in dynamic regions like Latin America, understanding the nuances of the PMA process becomes essential.
This article delves into the key components of PMA submissions, effective strategies for engaging with the FDA, and the common challenges faced by manufacturers, providing a roadmap for successfully bringing innovative medical devices to market while adhering to stringent regulatory standards.
Understanding Premarket Approval (PMA) in Medical Devices
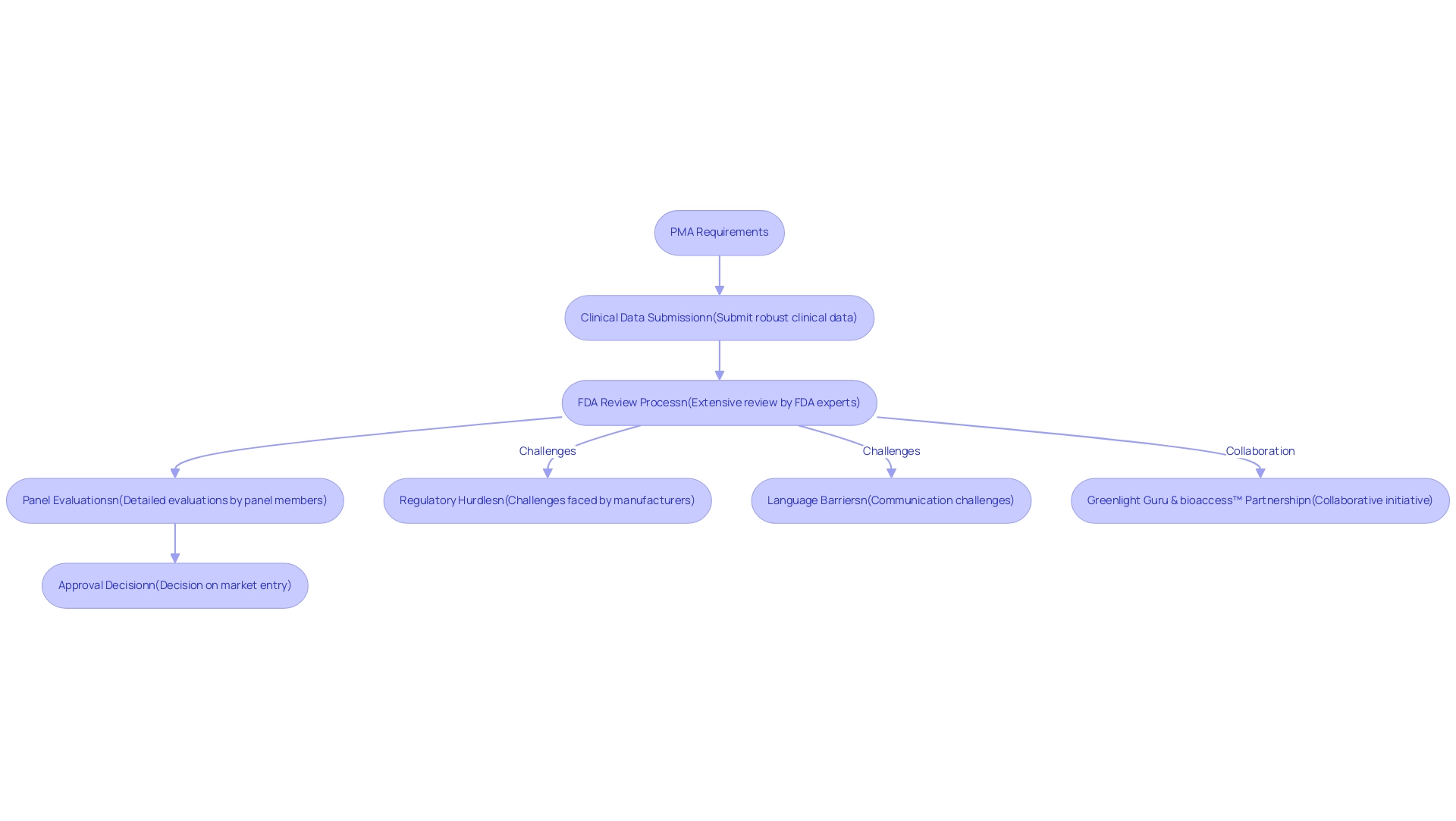
The PMA requirements constitute a vital regulatory pathway established by the FDA, specifically designed for medical device manufacturers to validate the safety and effectiveness of their products prior to market entry. This procedure is primarily required for high-risk devices, classified as Class III under the FDA classification system. Unlike the 510(k) pathway, which permits a more streamlined approval based on substantial equivalence to existing devices, the PMA method demands a more rigorous approach.
Manufacturers must fulfill the PMA requirements by presenting robust clinical data to demonstrate the device's safety and efficacy, undergoing an extensive review that includes detailed evaluations by FDA experts. Notably, Panel Track wait times have increased from an average of 285.80 days in 2023 to 289.62 days in 2024, highlighting the evolving regulatory landscape and its implications for manufacturers. As Medtech companies in Latin America encounter unique challenges such as regulatory hurdles, language barriers, and fragmented resources, collaboration becomes essential.
Initiatives like the partnership between Greenlight Guru and bioaccess™ aim to accelerate Medtech innovations and streamline clinical trials in the region, as illustrated by PAVmed's successful first-in-human study in Colombia. Comprehensive service capabilities, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
are crucial for assisting manufacturers in navigating the PMA requirements. Additionally, a survey of FDA Device Panel Members revealed that while 46% believed pivotal trials were well-designed, a significant 89% suggested that the FDA consult panel members on trial design and device labels.
This feedback underscores the complexities and challenges manufacturers face in navigating this regulatory pathway, emphasizing the importance of collaboration and informed trial design for Medtech companies in Latin America. For manufacturers, a comprehensive understanding of the PMA requirements is essential to successfully navigate the complex regulatory landscape, ensuring that innovative medical devices can be brought to market in compliance with stringent safety standards.
Key Components and Requirements for PMA Submissions
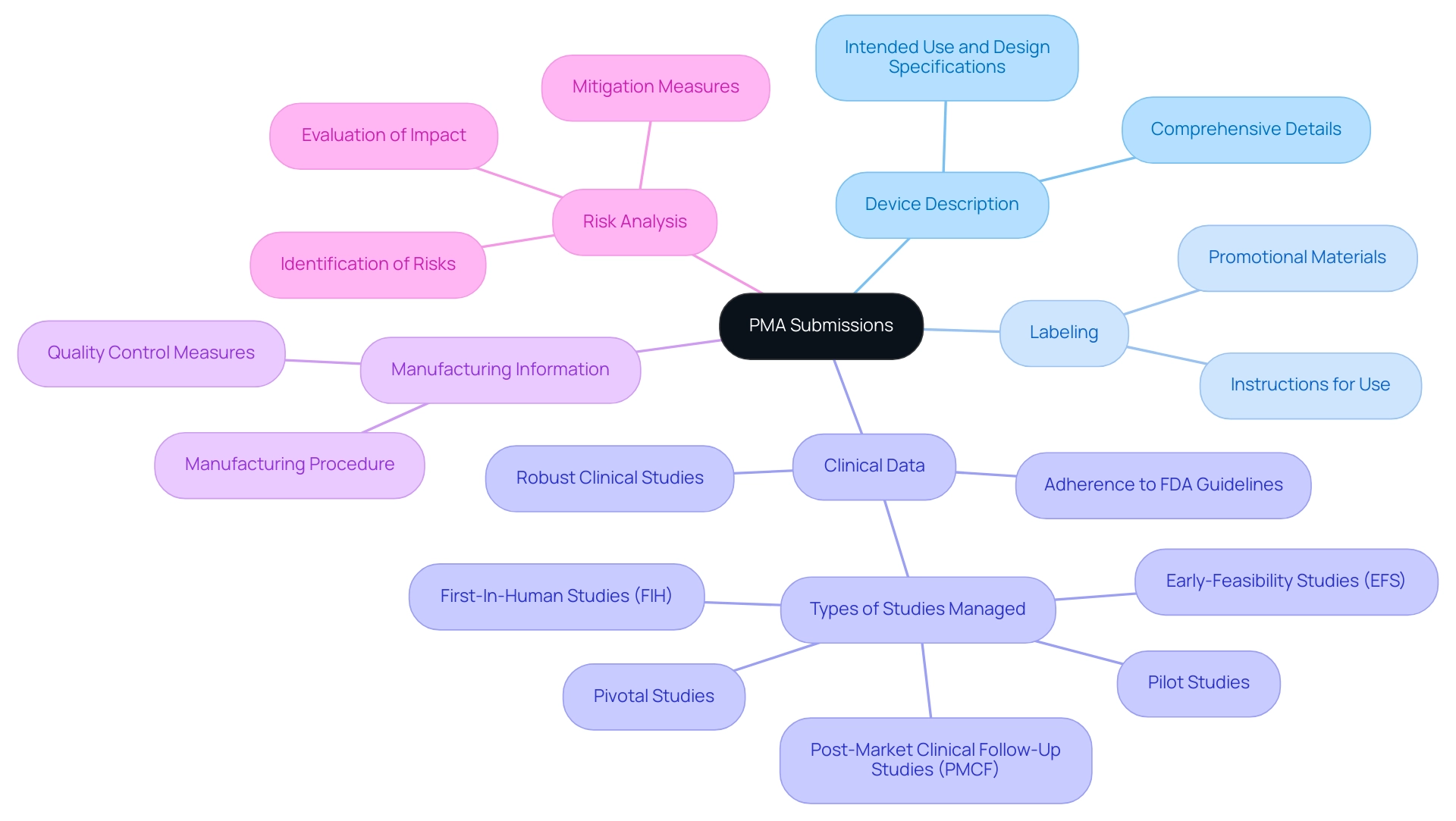
A successful PMA application depends on several essential elements, each contributing significantly to meeting PMA requirements and facilitating an efficient review. These key components include:
-
Device Description: This section must provide comprehensive details about the device, including its intended use, design specifications, and individual components.
A clear and detailed description aids reviewers in understanding the device's functionality and purpose.
-
Labeling: Proposed labeling is essential, encompassing instructions for use and any promotional materials. Proper labeling not only informs users but also helps in delineating the device's intended applications.
-
Clinical Data: Robust clinical studies are paramount, as they must demonstrate the device's safety and effectiveness. The data should be derived from well-designed trials that adhere to FDA guidelines, showcasing how the device performs under various conditions. Leveraging comprehensive clinical trial management services such as feasibility studies, site selection, and compliance reviews can enhance the quality of clinical data gathered.
bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring a thorough approach to clinical data collection.
-
Manufacturing Information: This component should outline the manufacturing procedure, including quality control measures and facility details. Transparent manufacturing information helps ensure that the device can be consistently produced in compliance with regulatory standards.
-
Risk Analysis: A thorough assessment of potential risks associated with the device is crucial. This analysis should include identification of risks, evaluation of their impact, and the measures taken to mitigate these risks.
In addition to these components, it is essential to understand the four types of PMAs, each with different applications and advantages. This knowledge can greatly impact the method adopted during the delivery phase.
As Chris Rush, a skilled biomedical engineer with vast experience overseeing clinical studies for Class III devices, emphasizes, effective communication throughout the procedure is essential. He notes,
'Essentially, communicating early and often will ease the situation for both sides and hopefully reduce any delays in what is already a fairly lengthy endeavor.'
His insights emphasize the significance of practical experience in managing the complexities of PMA applications.
Additionally, recognizing the knowledge of experts such as Katherine Ruiz, who focuses on Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, can offer essential guidance during the application journey. The adaptability and specialized expertise of the bioaccess® team are essential in navigating these complexities and ensuring successful results.
Furthermore, the recent advancement regarding Humanitarian Use Devices, which are exempt from standard PMA criteria under specific conditions, is critical in comprehending the regulatory landscape and potential exemptions that may influence PMA applications.
Finally, grasping the latest PMA requirements for 2024 is vital for optimizing application results. This proactive approach not only enhances the likelihood of approval but also contributes to the larger community of over 200,000 medical device professionals striving to outperform their peers.
Navigating the PMA Submission Process: Steps and Procedures
The PMA submission process involves several critical steps that require careful attention and preparation:
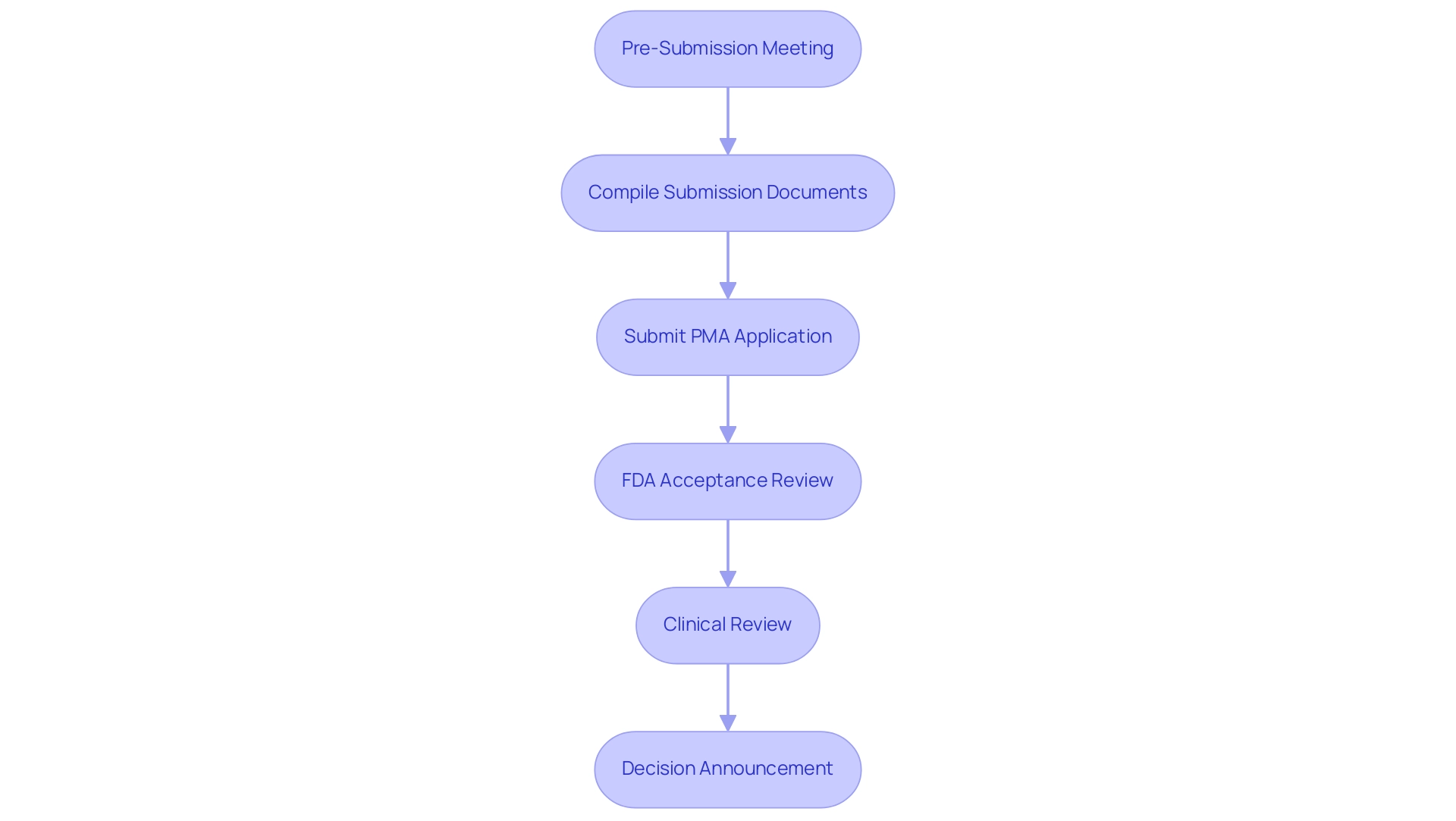
- Pre-Submission Meeting: Initiating a dialogue with the FDA is essential for discussing the strategy for proposals and obtaining valuable feedback on suggested studies. This meeting can be requested within 100 days of filing the PMA, emphasizing the importance of early engagement for an initial assessment of the review status.
- Compile Submission Documents: It is vital to gather all necessary documentation and data, ensuring that each component aligns with FDA guidelines for completeness and compliance.
- Submit PMA Application: The application must be sent through the FDA's electronic submission gateway, adhering to the latest protocols.
- FDA Acceptance Review: After the application is sent, the FDA performs a preliminary acceptance review to ensure that it is complete and prepared for further evaluation.
- Clinical Review: During this phase, the FDA thoroughly assesses the clinical data provided, which may lead to requests for additional information or clarifications as needed.
- Decision Announcement: Ultimately, the FDA will announce its decision, which may result in approval, a request for further studies, or denial of the application. Navigating these steps with diligence and preparation is crucial for fulfilling the PMA requirements for a successful application. As noted by Ellie Reynolds, Director of Regulatory Affairs and Quality Assurance, effective navigation of the PMA requirements during the submission procedure is integral to ensuring compliance and success in medical device approvals.
Furthermore, it is important to consider that the FDA can withdraw approval of a PMA if certain grounds under the FD&C Act apply or if the PMA requirements are not met, highlighting the consequences of non-compliance. Furthermore, factors like compliance reviews and project management play a significant role in the overall regulatory environment and can impact the PMA requirements framework. Applicants may also qualify for a Small Business fee, which can significantly influence financial considerations during the PMA.
Comprehending these steps, along with recent alterations in FDA PMA application procedures, can greatly affect the result of the filing.
Engaging with the FDA: Strategies for Successful PMA Submissions
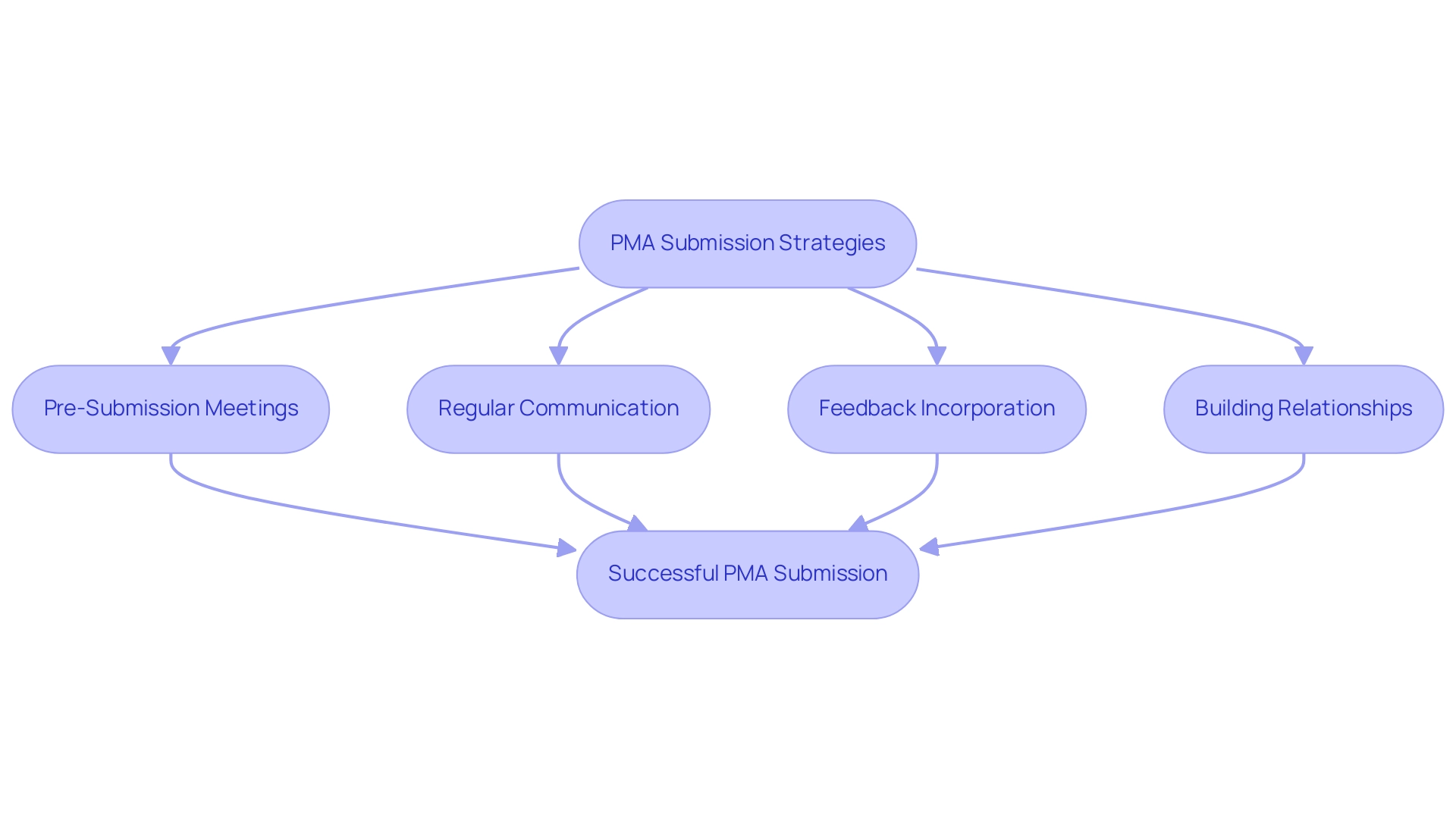
To enhance the likelihood of a successful PMA proposal, it is essential to implement effective strategies that comply with the PMA requirements when interacting with the FDA. Here are some key approaches:
-
Pre-Submission Meetings: Arrange these discussions to clarify the specific requirements and expectations from the FDA, which can significantly streamline the workflow.
Recent FDA guidance highlights the significance of these interactions in preparing a comprehensive proposal.
-
Regular Communication: Establish and maintain open lines of communication with FDA reviewers throughout the review process.
This ensures that any questions or concerns can be promptly addressed, enhancing the overall clarity and quality of the document.
-
Feedback Incorporation: Actively integrate feedback received from the FDA into your documentation.
This demonstrates responsiveness and adaptability, which can positively influence the PMA requirements for the review outcome.
-
Building Relationships: Cultivating relationships with FDA staff can facilitate a smoother review experience, providing insights that may not be readily available through formal channels.
These strategies are reinforced by industry experts, including Ana Criado, Director of Regulatory Affairs and an esteemed consultant with extensive experience in PMA requirements for medical device regulation, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia.
According to Quality Smart Solutions, "This evidence must be presented in a comprehensive package with detailed documentation of the device’s design, manufacturing method, labeling, and intended use," which underscores the PMA requirements for thorough presentations.
Additionally, leveraging solutions like DocShifter's Automated Report Level Publishing Solution can streamline document preparation by automating the merging of various document types into a compliant PDF.
This solution not only guarantees that all necessary documentation is thorough and properly formatted but also meets the PMA requirements for extensive documentation, ultimately accelerating the process for medical devices.
Significantly, small enterprises can gain from filling out FDA Form 3602, which may decrease the PMA filing fee by $331,000, further encouraging proactive interaction with regulatory bodies.
It is important to note that the PMA requirements typically involve a review period that can range from several months to over a year, making these strategies vital for timely and successful processes.
Applying these strategies not only increases the chances of a successful outcome but also aids in a more efficient and compliant approach.
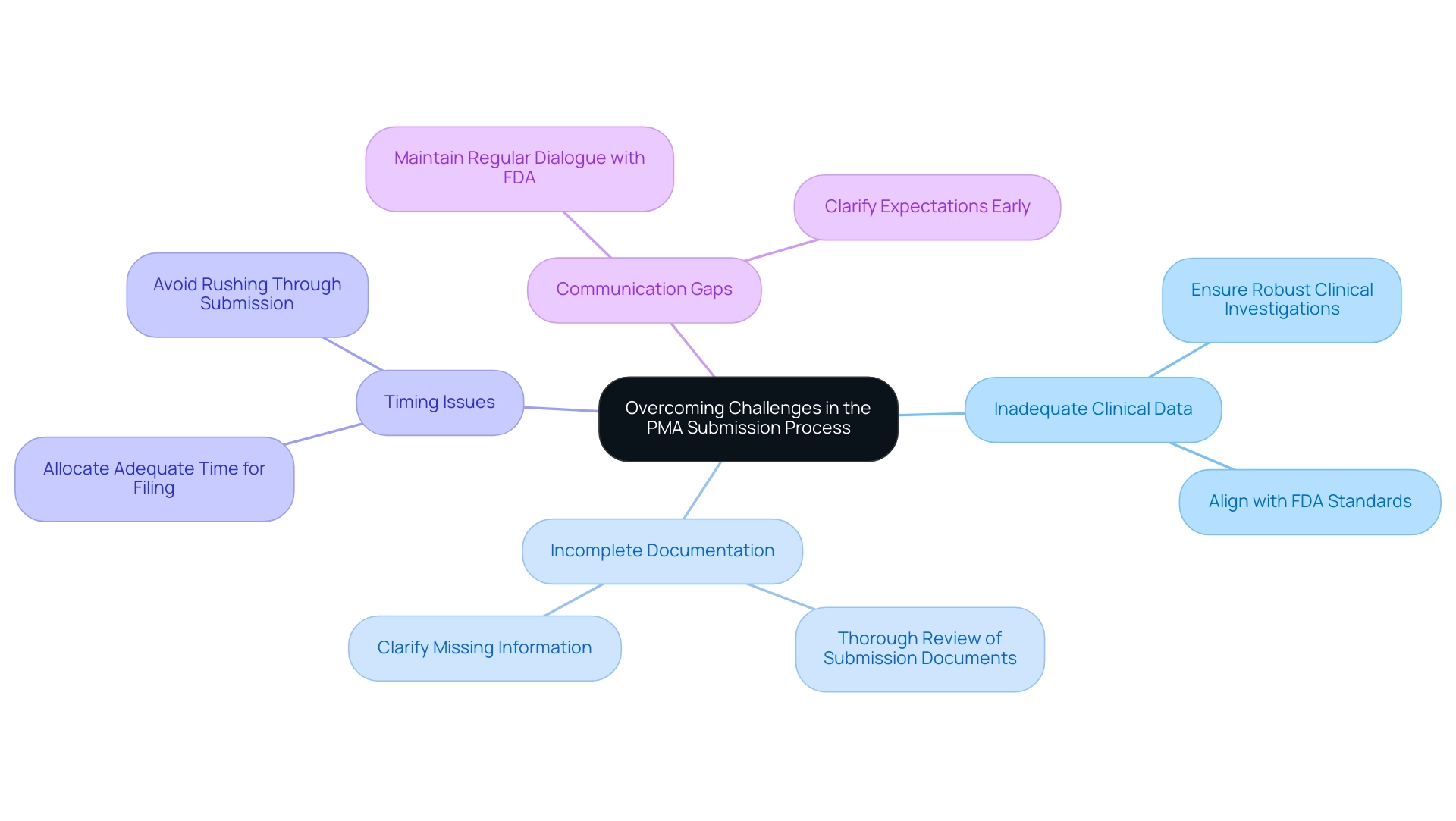
Overcoming Challenges in the PMA Submission Process
Navigating the PMA requirements during the filing journey presents several challenges that can hinder prompt approval for medical devices. Addressing these challenges proactively is essential for manufacturers aiming to fulfill the PMA requirements for successful proposals. The following are common hurdles encountered:
-
A significant concern in PMA applications is the inadequacy of clinical data, which can arise from poorly designed studies or insufficient sample sizes that do not meet PMA requirements. Ensuring that clinical investigations are robust and meet PMA requirements while aligning with FDA standards is crucial to demonstrating safety and efficacy. Recent reports suggest that among the 138 devices authorized for individuals over 21 years, problems with clinical evidence were often emphasized, highlighting the significance of sufficient clinical data in the evaluation.
bioaccess® specializes in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that your clinical trials are designed to meet these stringent requirements with the specialized knowledge and flexibility necessary for success in the Latin American market.
-
Incomplete Documentation: Thoroughly reviewing all submission documents is vital. Missing or unclear information related to PMA requirements can delay the review timeline and lead to requests for additional data, which prolongs time to market.
Dr. Akin Cil, an MD and board member of the American Shoulder and Elbow Surgeons, emphasizes that meticulous documentation is critical for meeting PMA requirements and ensuring a smoother review. At bioaccess®, our comprehensive support includes compliance reviews and project management to help streamline this crucial aspect of the submission.
-
Timing issues occur when manufacturers must allocate adequate time for the PMA requirements filing. Hurrying through this stage often leads to mistakes or unfinished entries, negatively affecting the approval timeline.
Communication Gaps: Maintaining consistent communication with FDA officials is critical. Regular dialogue can help clarify expectations and facilitate timely responses to any inquiries related to PMA requirements that may arise during the review process.
Our team, featuring Regulatory Affairs specialist Katherine Ruiz, is committed to guaranteeing that your communications are clear and effective, thus improving the chances of a smooth filing process.
A pertinent case study is the 'Toxicology Device Recall Analysis,' which involved 231 devices and indicated a moderate recall rate of 6.5%, with one class I recall (0.4%). The hazard ratio for recall was 0.58, indicating a lower risk of recall compared to the reference category. This demonstrates the real-world consequences of challenges encountered during PMA applications and the significance of efficiently addressing the PMA requirements.
Expert insights indicate that overcoming these obstacles not only improves the chances of a successful application but also aids in a smoother market entry for medical devices. By addressing these common challenges with effective strategies, manufacturers can significantly improve their PMA requirements and submission outcomes, leveraging the expertise and customized approach of bioaccess® in the context of Latin America.
Conclusion
Successfully navigating the Premarket Approval (PMA) process is crucial for medical device manufacturers, particularly in high-risk categories. The rigorous demands of the PMA pathway, including comprehensive clinical data and meticulous documentation, underscore the importance of a well-structured submission strategy. Engaging with the FDA through pre-submission meetings and maintaining open lines of communication can significantly enhance the likelihood of a successful outcome. Moreover, understanding the essential components of PMA submissions—ranging from device descriptions to risk analyses—provides a solid foundation for compliance.
Manufacturers face numerous challenges, including:
- Inadequate clinical data
- Incomplete documentation
- Communication gaps with regulatory bodies
Addressing these hurdles proactively is essential for timely approvals. By leveraging specialized services and fostering collaboration, particularly in dynamic regions like Latin America, companies can streamline their submissions and improve their chances of success.
Ultimately, a comprehensive understanding of the PMA process, coupled with effective engagement strategies and a commitment to high-quality submissions, empowers manufacturers to bring innovative medical devices to market. As the regulatory landscape continues to evolve, staying informed and prepared will be vital for achieving compliance and market success in the highly competitive field of medical technology.
Frequently Asked Questions
What is the PMA process and why is it important for medical device manufacturers?
The PMA (Premarket Approval) process is a regulatory pathway established by the FDA for medical device manufacturers to validate the safety and effectiveness of their products before they enter the market. It is primarily required for high-risk devices classified as Class III, demanding a more rigorous approach than the 510(k) pathway.
What are the main components required for a successful PMA application?
A successful PMA application includes several essential components: 1. Device Description: Comprehensive details about the device's intended use and design. 2. Labeling: Proposed labeling that includes instructions for use and promotional materials. 3. Clinical Data: Robust clinical studies demonstrating safety and effectiveness. 4. Manufacturing Information: Details about the manufacturing process and quality control measures. 5. Risk Analysis: Assessment of potential risks associated with the device and mitigation strategies.
How has the PMA review timeline changed recently?
The average wait time for PMA applications has increased from 285.80 days in 2023 to 289.62 days in 2024, indicating evolving regulatory challenges for manufacturers.
What initiatives are being implemented to assist Medtech companies in Latin America with PMA requirements?
Initiatives like the partnership between Greenlight Guru and bioaccess™ aim to accelerate Medtech innovations and streamline clinical trials in Latin America, addressing unique challenges such as regulatory hurdles and language barriers.
What role does clinical data play in the PMA process?
Clinical data is crucial for demonstrating the safety and effectiveness of the device. It must come from well-designed trials that adhere to FDA guidelines, and comprehensive clinical trial management services can help enhance the quality of this data.
What feedback do FDA Device Panel Members have regarding trial design and device labeling?
A survey revealed that while 46% of panel members believed pivotal trials were well-designed, a significant 89% suggested that the FDA should consult panel members on trial design and device labels, highlighting the complexities manufacturers face.
What recent advancements have been made regarding Humanitarian Use Devices in relation to PMA criteria?
Recent advancements indicate that Humanitarian Use Devices may be exempt from standard PMA criteria under specific conditions, which is important for understanding potential exemptions in the regulatory landscape.
Why is effective communication important during the PMA application process?
Effective communication throughout the PMA application process is essential, as it can help ease the situation for both manufacturers and the FDA, potentially reducing delays in the lengthy approval process.




