Overview
Regulatory requirements for clinical trials are essential for ensuring participant safety, data integrity, and the overall credibility of research results. The article outlines various key processes, such as feasibility studies, compliance reviews, and the necessity of informed consent, emphasizing that adherence to these regulations is vital for conducting ethical and effective clinical research.
Introduction
In the intricate world of clinical trials, regulatory requirements serve as the backbone for ensuring the safety and efficacy of new treatments. As the landscape of clinical research becomes increasingly complex, understanding these regulations is vital for researchers and participants alike.
This article delves into the foundational principles that govern clinical trials, exploring the essential guidelines, documentation, and ethical considerations that underpin successful research. From the critical role of regulatory authorities to the importance of informed consent, the discussion highlights the multifaceted nature of compliance in clinical trials.
By examining recent trends and case studies, this article aims to provide a comprehensive overview of how adherence to regulatory frameworks not only protects participants but also enhances the credibility and reliability of clinical research outcomes.
Foundations of Regulatory Requirements in Clinical Trials
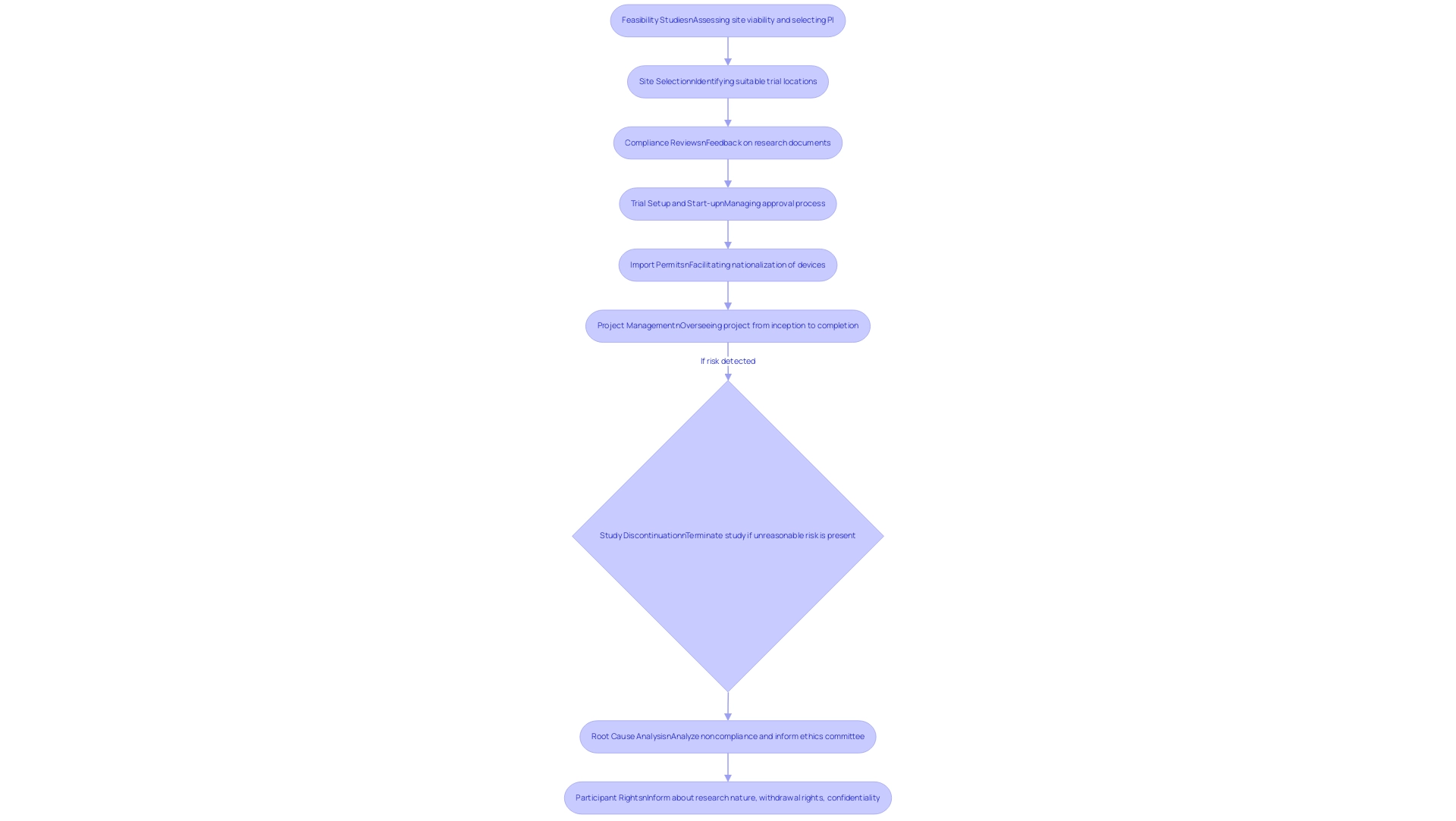
Regulatory requirements for clinical trials play a crucial role in safeguarding the safety and efficacy of emerging treatments. Our comprehensive clinical study management services encompass:
- Feasibility Studies: Assessing the viability of the research site and selecting a qualified principal investigator (PI).
- Site Selection: Identifying suitable locations for conducting trials.
- Compliance Reviews: Providing detailed feedback on research documents to ensure compliance with country-specific requirements.
- Trial Setup and Start-up: Managing the approval process with ethics committees and health ministries.
- Import Permits: Facilitating the nationalization of investigational devices.
- Project Management: Overseeing the research project from inception to completion, including monitoring and reporting on project status, inventory, and adverse events (both serious and non-serious).
This structured approach ensures compliance with the regulatory requirements for clinical trials, prioritizing participant protection, data integrity, and the overall credibility of trial results. Notably, if a study presents unreasonable risk to participants, the sponsor is mandated to discontinue the study within five working days following this determination, in compliance with the regulatory requirements for clinical trials, reflecting our commitment to participant safety.
Katherine Ruiz, an expert in compliance matters for medical devices and in vitro diagnostics in Colombia, leads our efforts in ensuring adherence to local and international standards. As Daniel Hernandez, NIH Regulations Officer, noted, 'NIH is completing a multiyear initiative to modernize the ClinicalTrials.gov website to deliver an improved user experience on an updated platform that enhances efficiency.' This modernization highlights a broader trend in compliance, ensuring that trial execution meets current standards while evolving with the needs of stakeholders.
Additionally, health insurers increasingly rely on customers not appealing denials, often described as 'rationing by inconvenience,' showcasing the implications of regulatory compliance. A pertinent case study, titled 'Premature Study Termination Protocol,' illustrates that according to 21CFR312 and US-ICH-GCPS, the sponsor must adhere to the regulatory requirements for clinical trials by discontinuing the study and notifying relevant parties within five working days if it presents significant risk to participants. This emphasizes the necessity of conducting a root cause analysis for noncompliance with regulatory requirements for clinical trials and informing the ethics committee of the reasons for termination.
Furthermore, participants have the right to be informed about the nature and purpose of the research, their rights to withdraw, and the confidentiality of their records, as mandated by the regulatory requirements for clinical trials and ethical standards outlined in 21CFR50 and related regulations. A thorough grasp of these fundamental principles, along with our wide-ranging service capabilities, is crucial for meeting regulatory requirements for clinical trials and executing successful research studies.
Key Guidelines and Documentation for Clinical Trial Compliance
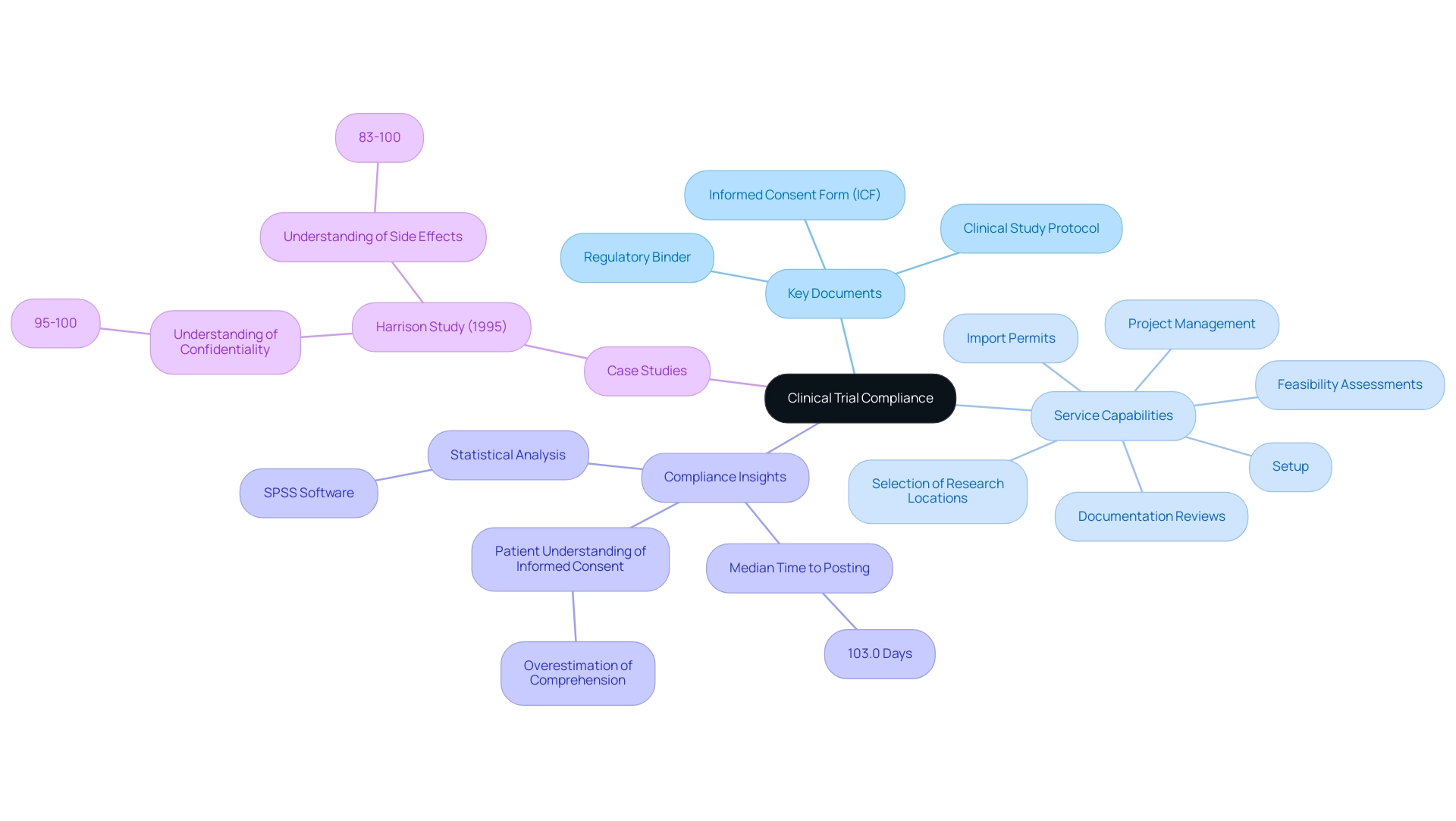
In clinical study Regulatory Affairs, ensuring compliance with the regulatory requirements for clinical trials hinges on several key documents, notably the informed consent form (ICF), clinical study protocol, and the regulatory binder. Our service capabilities include:
- Feasibility assessments
- Selection of research locations and principal investigators (PI)
- Setup
- Import permits
- Project management
- Comprehensive reviews and feedback on documentation to meet country requirements
The ICF is essential as it provides participants with comprehensive information about the project's purpose, procedures, potential risks, and benefits, enabling them to make informed decisions regarding their involvement.
Recent insights indicate that compliance rates for research documentation are on the rise in 2024, with a median time to posting reduced to 103.0 days, underscoring the importance of meticulous documentation in maintaining ethical standards. The clinical protocol outlines the research design and methodologies, serving as a roadmap for the research team, while the compliance binder functions as a crucial repository of important documents that confirm adherence to standards. Katherine Ruiz, an expert in compliance matters for medical devices and in vitro diagnostics in Colombia, leads efforts to ensure adherence and effective management throughout the trial process.
As Peter Doran notes, PD leads the research team, secured funding for the project, contributed to the design of the research and reviewed the manuscript. This collaborative effort emphasizes the necessity of organized documentation, which is crucial for successful audits and meeting regulatory requirements for clinical trials. Furthermore, studies like the Harrison Study (1995), which assessed informed consent understanding among 175 adult patients in an HIV vaccine study, revealed that participants had a high comprehension of confidentiality (95-100%) and side effects (83-100%).
However, it is important to recognize that patients often overestimate their comprehension of informed consent, which may weaken the ethical foundations of research studies and medical practice. Such discoveries emphasize the continued necessity to prioritize informed consent in research studies to maintain ethical standards and participant trust. Additionally, statistical analysis was performed using SPSS software, further emphasizing the importance of data integrity in compliance.
The Role of Regulatory Authorities in Clinical Trial Oversight
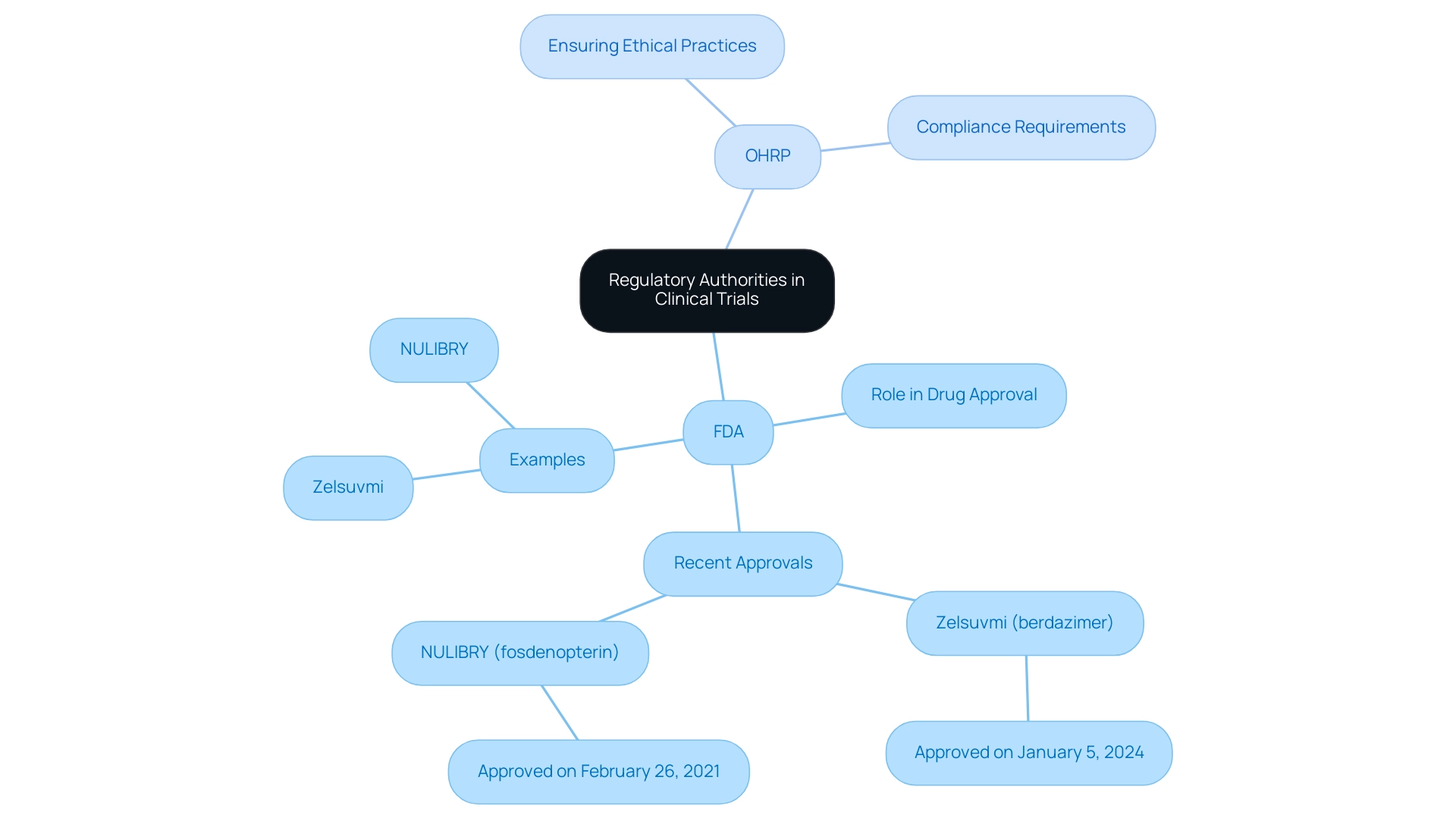
Regulatory bodies like the Food and Drug Administration (FDA) and the Office for Human Research Protections (OHRP) play essential roles in supervising research studies, ensuring that they meet the regulatory requirements for clinical trials to guarantee safety and effectiveness through thorough assessments. The FDA is responsible for assessing new pharmaceuticals and medical devices before they reach the market, exemplified by the recent approval of Zelsuvmi (berdazimer) for molluscum contagiosum on January 5, 2024. This approval highlights the FDA's commitment to advancing medical treatments while prioritizing patient safety.
Concurrently, the OHRP ensures ethical practices in research involving human subjects, mandating compliance with established ethical guidelines. These organizations offer essential direction on compliance submissions and oversee adherence to the regulatory requirements for clinical trials, performing inspections to guarantee that studies meet all required standards. As of November 2024, there are 515,319 registered medical trials worldwide, with 283,981 carried out outside the U.S., highlighting the vast extent of medical research and the crucial requirement for strong governance.
Moreover, NULIBRY (fosdenopterin) received approval on February 26, 2021, to mitigate the risk of death in MoCD Type A, demonstrating the FDA's responsiveness to urgent health issues. For medical researchers in Latin America, comprehending and interacting with these governance frameworks is essential for effectively navigating the regulatory requirements for clinical trials. This corresponds with the extensive research study management services provided—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
Medtech companies in the region often encounter challenges such as regulatory fragmentation and resource limitations, making it essential to collaborate with experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices in Colombia, to bridge the gaps between innovation and execution in the region.
Ethical Considerations and Participant Protection in Clinical Trials
Ethical considerations in research studies are of utmost importance, particularly in the realms of informed consent and the safeguarding of vulnerable populations. Researchers are ethically required to obtain voluntary and informed consent from participants, ensuring they have a clear understanding of the project's risks and benefits. This is particularly critical for vulnerable groups, such as children and individuals with cognitive impairments, whose rights and welfare must be prioritized throughout the research process.
A recent research effort aims to provide evidence on the proportion of clinical trial participants who understand informed consent components, highlighting the need for improvements in the informed consent process. Statistics reveal that:
- 24.9% of participants in developed countries grasped the concept of placebo
- 43.4% in developing nations
- 64% in the least developed countries
This disparity underscores the necessity for clear communication and comprehension regarding informed consent.
Furthermore, a case analysis titled 'Publication Bias Analysis' conducted on informed consent components revealed risks of bias in areas such as the purpose of the research and confidentiality. This demonstrates the importance of careful interpretation of study results and its potential impacts on informed consent understanding. Ethical oversight provided by Institutional Review Boards (IRBs) plays a pivotal role in maintaining participant protection and ensuring adherence to ethical standards throughout the research lifecycle.
As emphasized by Fatima N. Mirza,
The clarity of these forms is of utmost importance, perhaps now more than ever.
This statement emphasizes the necessity for additional inquiry into the readability of consent forms and its relationship with participant retention, reinforcing the call for improved ethical guidelines and practices in research.

Navigating Changes in Clinical Trial Regulations
The landscape of medical study regulations is in a state of continuous evolution, underscoring the necessity for researchers to remain vigilant regarding recent updates and changes. Our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance assessments
- Study setup
- Import permits and nationalization of investigational devices
- Project oversight
- Reporting (study status, inventory, serious and non-serious adverse events)
This ensures streamlined processes that align with the latest compliance requirements. In 2023, the FDA revised its stance on the use of electronic systems, records, and signatures, aiming to enhance the approval process for innovative therapies.
This change is especially important as the incorporation of Real-World Evidence (RWE) into oversight submissions is gaining momentum, mirroring a wider trend towards openness and effectiveness in medical study data reporting. Technology and patient engagement are central to this evolution, as they enhance the ability to collect and analyze data effectively. Recent statistics indicate that:
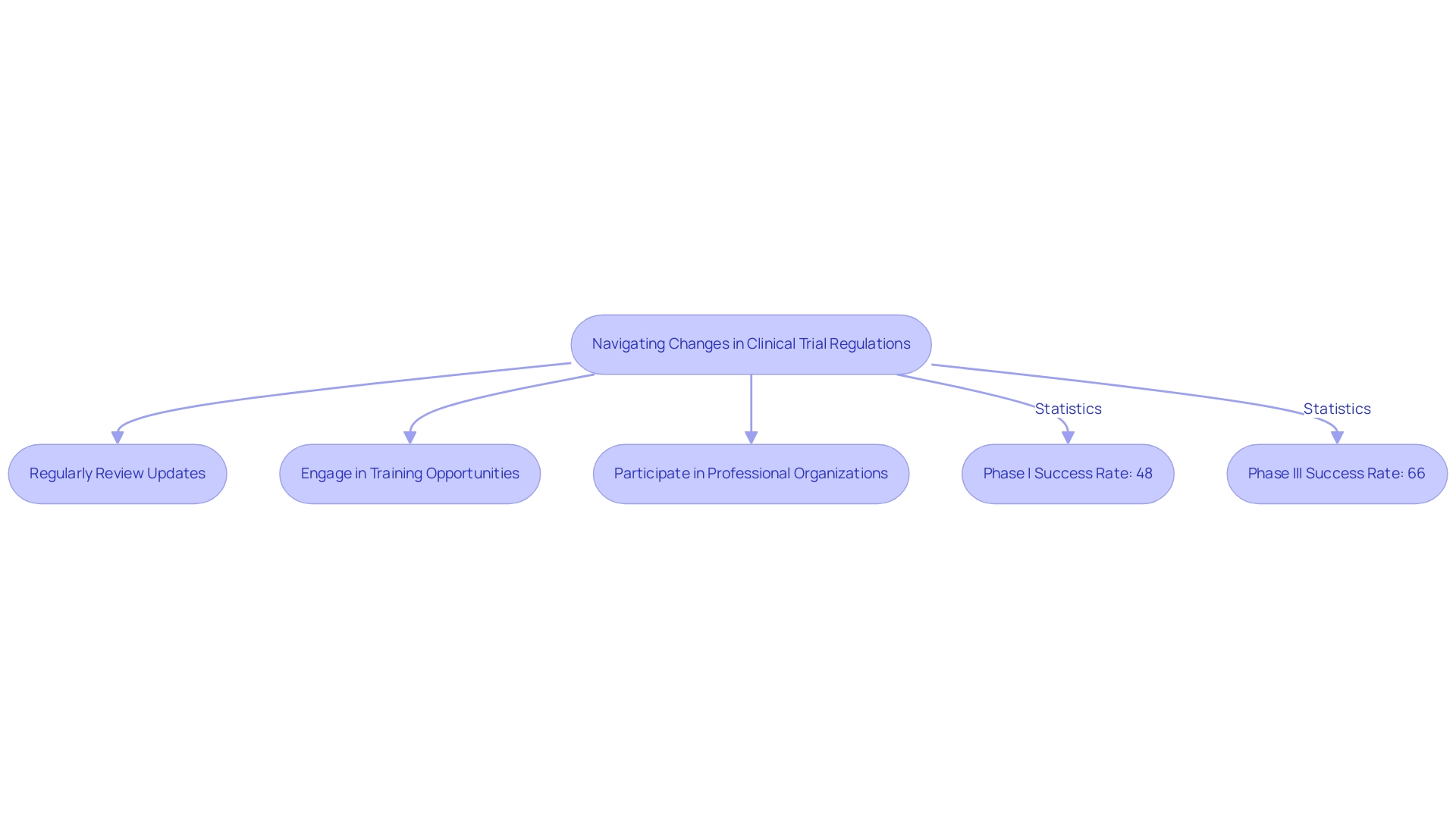
- Phase I success rates have risen to 48%
- Phase III rates have reached 66%, surpassing the 10-year pre-pandemic average of 56%
This rise in success rates is a promising sign of the industry's momentum and adaptability in the face of policy changes. As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, remarked, 'I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.'
Furthermore, Walgreens has demonstrated a strong dedication to research studies, signing 15 agreements for participant recruitment in Q3 of 2023, indicating a growing momentum in collaborations with retail pharmacies to access a larger pool of potential study participants. To effectively navigate the regulatory requirements for clinical trials and maintain compliance, researchers must:
- Regularly review updates
- Engage in training opportunities
- Actively participate in professional organizations
Leveraging the expertise of leaders like Katherine Ruiz in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia.
Conclusion
The intricate framework of regulatory requirements in clinical trials is essential for safeguarding participant safety and ensuring the efficacy of new treatments. This article has explored the foundational principles that govern clinical trials, emphasizing the critical roles of regulatory authorities, the importance of thorough documentation, and the ethical considerations that must be adhered to throughout the research process.
Key guidelines, such as the informed consent form and clinical trial protocol, serve as cornerstones for compliance, enabling participants to make informed decisions while ensuring that researchers follow established standards. The evolving landscape of clinical trial regulations further underscores the necessity for researchers to remain informed about changes and advancements, particularly as technology and patient engagement play increasingly significant roles in data collection and analysis.
Ultimately, adherence to regulatory frameworks not only protects participants but also enhances the credibility and reliability of clinical research outcomes. By prioritizing compliance and ethical practices, researchers can contribute to the advancement of medical science while fostering trust and integrity in the clinical trial process. The commitment to rigorous oversight and participant protection will continue to be paramount as the field navigates the complexities of modern clinical research.
Frequently Asked Questions
What are the main components of clinical study management services?
The main components include feasibility studies, site selection, compliance reviews, trial setup and start-up, import permits, and project management.
What is the purpose of feasibility studies in clinical trials?
Feasibility studies assess the viability of the research site and help in selecting a qualified principal investigator (PI).
How does compliance reviews contribute to clinical trials?
Compliance reviews provide detailed feedback on research documents to ensure they meet country-specific regulatory requirements.
What is involved in the trial setup and start-up process?
The trial setup and start-up process involves managing the approval process with ethics committees and health ministries.
What role do import permits play in clinical trials?
Import permits facilitate the nationalization of investigational devices necessary for the trials.
What does project management entail in the context of clinical trials?
Project management includes overseeing the research project from inception to completion, monitoring and reporting on project status, inventory, and adverse events.
What happens if a study presents unreasonable risk to participants?
If a study presents unreasonable risk, the sponsor is required to discontinue the study within five working days following this determination.
Who leads the efforts to ensure compliance with regulatory standards in clinical trials?
Katherine Ruiz, an expert in compliance matters for medical devices and in vitro diagnostics in Colombia, leads these efforts.
What is the significance of the informed consent form (ICF) in clinical trials?
The ICF provides participants with comprehensive information about the project’s purpose, procedures, potential risks, and benefits, enabling informed decision-making regarding their involvement.
How are compliance rates for research documentation trending in 2024?
Compliance rates for research documentation are on the rise, with a median time to posting reduced to 103.0 days.
What key documents are essential for ensuring compliance with regulatory requirements for clinical trials?
Key documents include the informed consent form (ICF), clinical study protocol, and the regulatory binder.
What does the clinical study protocol outline?
The clinical study protocol outlines the research design and methodologies, serving as a roadmap for the research team.
Why is organized documentation important in clinical trials?
Organized documentation is crucial for successful audits and meeting regulatory requirements for clinical trials.
What did the Harrison Study (1995) reveal about informed consent understanding?
The Harrison Study revealed that participants had a high comprehension of confidentiality and side effects, but often overestimated their understanding of informed consent.
How is data integrity emphasized in compliance with clinical trial regulations?
Data integrity is emphasized through meticulous documentation and statistical analysis, ensuring adherence to ethical standards.

