Overview
The article delves into the intricate regulatory requirements governing clinical trials in Latin America, underscoring the specific frameworks, advantages, and challenges encountered by researchers across various countries. It asserts that a comprehensive understanding of these regulations, particularly Brazil's recent legislative changes and Colombia's competitive advantages, is essential for adeptly navigating the landscape and optimizing clinical research outcomes in the region.
Introduction
Latin America is rapidly emerging as a pivotal player in the global clinical trials landscape. However, navigating its intricate regulatory framework can be a daunting task for researchers and sponsors alike. Each country boasts its own set of laws and guidelines, creating a complex tapestry that varies from Brazil's streamlined approval processes to Argentina's more stringent requirements. Amidst these challenges, Colombia stands out as a beacon of opportunity, offering not only cost-effective solutions but also a robust healthcare system and an ambitious innovation strategy.
As the region undergoes significant reforms aimed at enhancing efficiency and transparency, understanding the nuances of this evolving environment is essential for successful trial execution. This article delves into the regulatory dynamics, recruitment strategies, and emerging trends shaping clinical research in Latin America, providing valuable insights for stakeholders looking to capitalize on this burgeoning market.
Navigating the Regulatory Landscape for Clinical Trials in Latin America
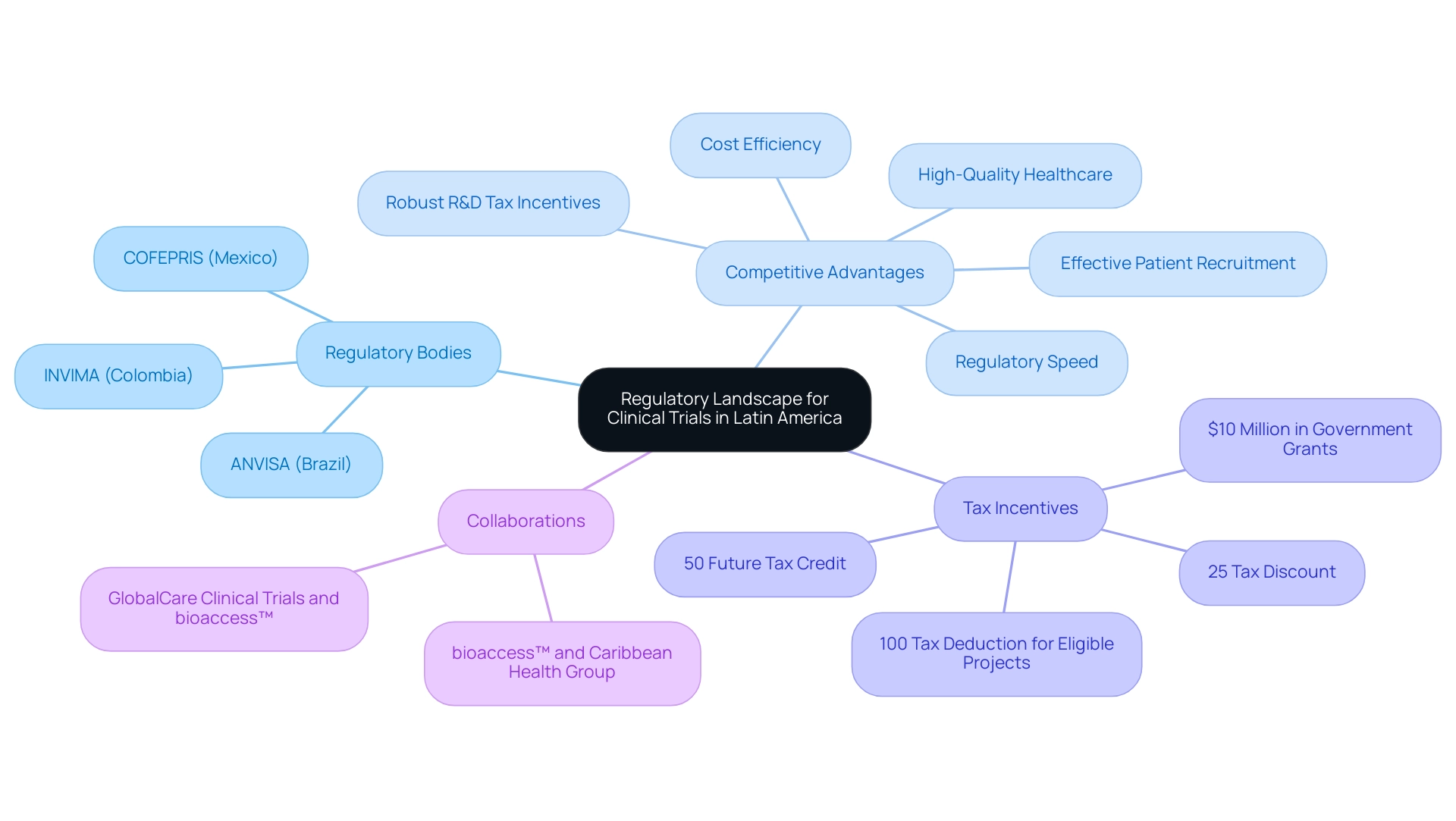
The oversight framework for medical studies in Latin America is substantially shaped by the regulatory requirements governing trials in the region, revealing a complex landscape of laws and guidelines that differ markedly among countries, each overseen by its respective governing body. For example, Brazil's ANVISA and Mexico's COFEPRIS are pivotal in establishing the compliance framework necessary to meet these regulatory demands, ensuring that both local and international standards are adhered to. Notably, Colombia has positioned itself as a premier destination for first-in-human studies, leveraging several competitive advantages:
- Cost efficiency
- Regulatory speed
- High-quality healthcare
- Effective patient recruitment
- Robust R&D tax incentives
Savings in Colombia can exceed 30% when compared to similar studies conducted in North America or Western Europe. Furthermore, the total review process involving the IRB/EC and the Ministry of Health (INVIMA) is remarkably swift, typically taking only 90-120 days. The World Health Organization ranks Colombia's healthcare system as #22 globally, and with a population exceeding 50 million, the country boasts a well-covered patient pool for recruitment, with approximately 95% of individuals under universal healthcare. Additionally, investments in science and innovation benefit from substantial tax deductions, including:
- A 100% tax deduction for eligible projects
- A 25% tax discount
- A 50% future tax credit
- Around $10 million in government grants
This creates a compelling environment for research.
Significantly, collaborations such as that between bioaccess™ and Caribbean Health Group aim to establish Barranquilla as a leading hub for medical studies, supported by the Colombian Minister of Health. Similarly, partnerships like that of GlobalCare Clinical Trials with bioaccess™ have showcased remarkable enhancements in recruitment efficiency, achieving over a 50% reduction in recruitment time while retaining 95% of participants. As Julio G. Martinez-Clark, CEO, remarked, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.'
Understanding these dynamics and adeptly navigating the governance structure with local expertise will be crucial for success in this burgeoning center for medical studies.
Country-Specific Regulatory Frameworks: A Comparative Analysis
The regulatory environment for research studies in Latin America is intricately shaped by the regulatory requirements for trials in the region, presenting investigators with a diverse array of frameworks to navigate. Comprehensive study management services are paramount for success. Notably, Brazil's Law 14.874/24, enacted in 2024, has streamlined the trial approval process, empowering local ethics committees to independently approve protocols and expediting the initiation of studies. As we look to 2025, this legislative change is poised to significantly reduce approval times, making Brazil an increasingly attractive destination for clinical research.
In stark contrast, if a project necessitates review by CONEP, the deadlines are stringent: 15 days for document validation and 45 days for ethical assessment. Argentina, on the other hand, maintains a more rigorous oversight environment, requiring comprehensive documentation and extended approval timelines. As of 2025, compliance requirements in Argentina continue to present challenges for sponsors, who must brace for a more demanding review process.
This contrast underscores the critical importance of understanding the regulatory requirements for Latin America trials when organizing research initiatives. Countries like Colombia and Chile are actively refining their oversight frameworks to foster a more supportive atmosphere for research. These nations are implementing changes aimed at streamlining procedures and enhancing international cooperation, which could further elevate their appeal for research studies. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, plays a pivotal role in navigating these regulatory requirements. A comparative analysis of the regulatory landscape reveals that while Brazil's recent legislative changes may facilitate quicker market access, the complexities inherent in Argentina's regulations necessitate meticulous planning and strategic foresight.
For instance, a recent survey highlighted a widespread lack of awareness among healthcare professionals and patients regarding the benefits of experimental studies, resulting in diminished participation rates in such evaluations. Addressing this knowledge gap through targeted educational initiatives is essential for enriching the research landscape across Latin America. Furthermore, sponsors are required to retain essential documents for a minimum of two years following the approval or discontinuation of a marketing application, and cancellations of research applications are final, with no possibility of reactivation—a fact that must be communicated to all involved parties.
In summary, grasping the regulatory requirements for Latin America trials, particularly the nuances of Brazil's Law 14.874/24, alongside leveraging comprehensive services from bioaccess—such as setup, project management, and reporting—is crucial for researchers aiming to optimize their locations and processes. This knowledge not only aids in compliance but also enhances the potential for successful study outcomes. Moreover, the impact of Medtech research on local economies, including job creation and healthcare enhancement, underscores the significance of these studies in fostering international cooperation and economic development.
As Gustavo Gössling observed, 'LACOG has created significant efforts aimed at enhancing and improving access to cancer research, which ultimately can lead to a rise in the rate of patient referrals by physicians and an increase in the number of physicians involved in cancer research.
The Role of Good Clinical Practice in Latin American Trials
Good Clinical Practice (GCP) is indispensable for the successful execution of clinical studies in Latin America, especially given the regulatory requirements for trials in the region. As bioaccess® emerges as a leading Contract Research Organization, it plays a pivotal role in supporting these initiatives. These guidelines are meticulously designed to ensure that studies are planned, conducted, and reported with a focus on the rights, safety, and well-being of participants. Adhering to GCP is not merely about compliance; it significantly enhances the credibility of findings, a critical factor in today's competitive global landscape.
In 2025, GCP compliance has gained increased prominence in Latin America, underscoring the regulatory requirements for trials in the region. Oversight organizations are intensifying their emphasis on strict adherence to these standards. It is essential to train all research personnel in GCP principles, ensuring that everyone involved comprehends the protocols and ethical considerations intrinsic to medical studies. This comprehensive training fosters trust among stakeholders—including oversight bodies, sponsors, and participants—ultimately facilitating smoother approval processes.
The impact of GCP compliance on clinical study outcomes is vital, particularly when considering the regulatory requirements for trials in Latin America. Research indicates that studies conducted under stringent GCP guidelines yield more reliable data, which is essential for meeting regulatory requirements and for the successful submission of New Drug Applications to regulatory agencies. For example, a New Drug Application is submitted to the FDA for review and approval once adequate testing has been completed, highlighting the critical nature of trustworthy data derived from studies conducted under GCP guidelines.
Cultural factors in Latin America, such as a heightened trust in physicians, can enhance patient participation in studies, resulting in more comprehensive data collection and improved study efficiency.
A noteworthy example is the collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a key research hub in Latin America. Supported by Colombia's Minister of Health, Juan Pablo Uribe, this initiative aims to create a conducive environment for medical studies, thereby increasing the region's attractiveness for health-related evaluations. The Minister's backing reflects a strong governmental commitment to establishing Colombia as a research center, thereby bolstering investor confidence and streamlining study initiation processes.
Additionally, GlobalCare Clinical Studies has partnered with bioaccess™ to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and impressive retention rates of 95%.
As the landscape of medical studies evolves, upholding high standards of GCP compliance remains crucial for meeting regulatory requirements in Latin America, ensuring that innovative solutions are brought to market effectively and ethically. The implications of GCP compliance extend beyond mere legal conformity; they are essential in shaping the future of medical research in the region, particularly concerning regulatory requirements for Latin America trials, fostering a culture of quality and trust vital for the success of healthcare innovations.
Challenges in Conducting Clinical Trials: Recruitment and Regulatory Hurdles
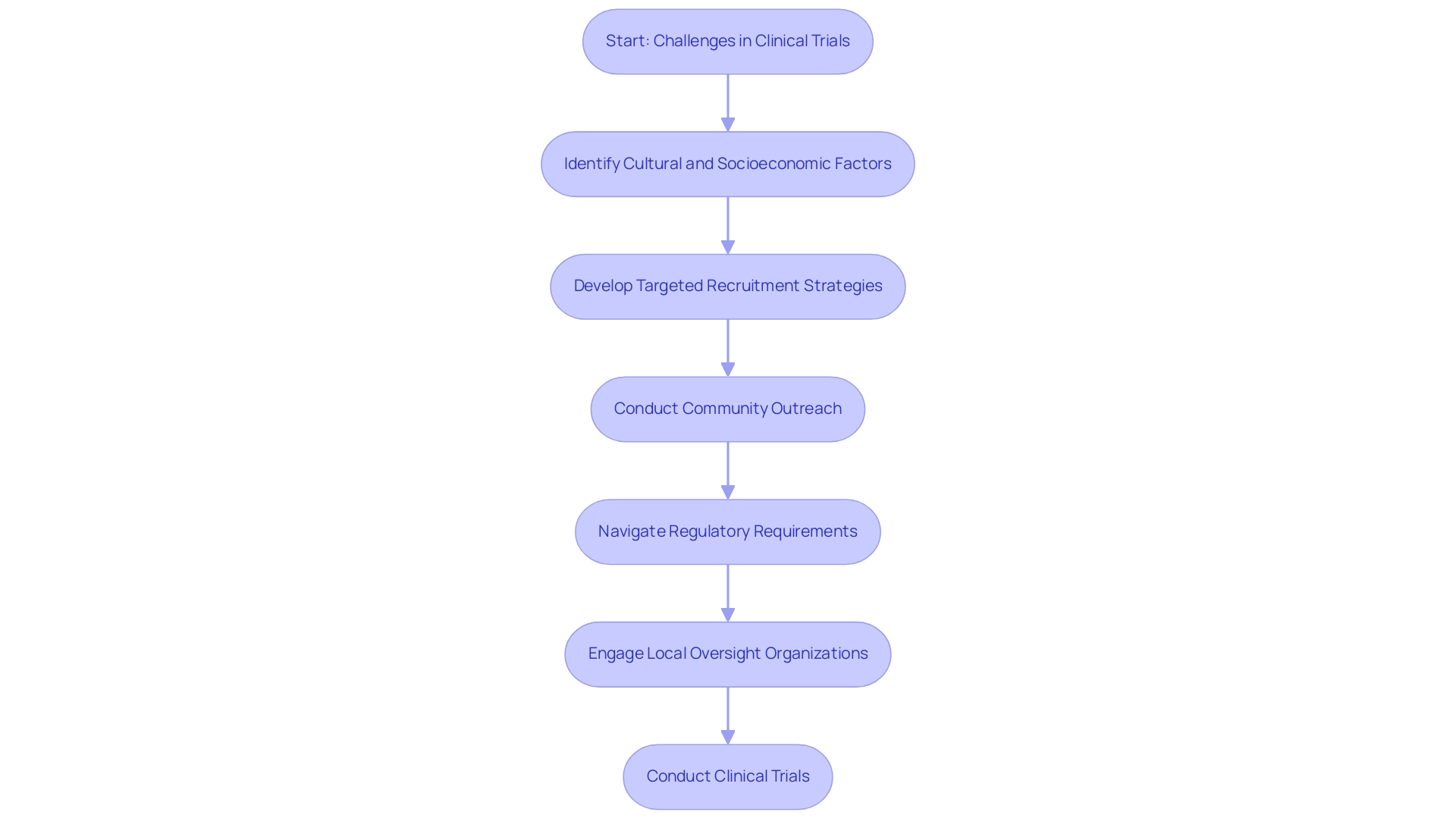
Carrying out research studies in Latin America presents unique challenges, particularly in participant recruitment and adherence to regulatory requirements for trials in the region. Recruitment efforts can be significantly influenced by cultural differences, socioeconomic disparities, and a general lack of awareness regarding the advantages of research studies among potential participants. Many communities may not fully understand the implications of participating in studies, leading to hesitancy or outright refusal.
To address these challenges, researchers must implement targeted recruitment strategies sensitive to local demographics and cultural contexts, potentially through community outreach initiatives that educate potential participants about the advantages of clinical trial involvement. As highlighted by FOMAT Medical Research, "Latin America provides significant diversity for swift recruitment; most nations are multicultural and have become an excellent place to conduct a wide-ranging study." Utilizing this multicultural diversity can improve recruitment efforts, allowing for a wider range of participants.
Navigating the regulatory requirements for trials in Latin America can also be intricate, as each nation presents its own set of compliance requirements, which can lead to delays in the start of the study. Bioaccess's service capabilities, including feasibility and selection of research sites, compliance reviews, setup, and project management, are crucial for facilitating smoother approval processes and enhancing overall efficiency. Building strong connections with local oversight organizations, such as INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential for tackling specific compliance challenges and ensuring adherence to health authority standards.
The setup process involves careful planning and coordination with regulatory bodies to ensure that the regulatory requirements for trials in Latin America are met before commencing the study. Bioaccess assists in navigating these processes, ensuring that all necessary documentation is prepared and submitted in a timely manner. Furthermore, the project management aspect includes continuous monitoring of the experiment's progress, management of study status, inventory tracking, and reporting of serious and non-serious adverse events, which are essential for maintaining the integrity of the experiment.
Moreover, the increasing focus on health equity and access to care in the region drives initiatives aimed at addressing local health challenges, which can encourage participation in research trials. The expected 1.9 million new cancer cases in the United States for 2022 highlights the pressing need for effective medical studies to address such health concerns. As the landscape evolves, it is essential for researchers to remain adaptable and informed about changing dynamics in medical research in Latin America, especially regarding regulatory requirements, to effectively overcome barriers to participant recruitment and regulatory compliance.
Furthermore, the effect of Medtech research studies on local economies cannot be ignored. These studies not only contribute to job creation and economic growth but also improve healthcare access and outcomes within the communities involved. However, it is important to be aware of potential conflicts of interest in patient recruitment, which can compromise the quality of studies and the health of participants.
A case study examining the misalignment of research objectives in medical studies emphasizes the importance of prioritizing ethical standards alongside commercial interests. By addressing these ethical considerations, researchers can enhance the integrity of their studies and contribute to the advancement of medical knowledge.
Emerging Trends and Positive Changes in Latin America's Regulatory Environment
The oversight environment for medical studies in Latin America is currently experiencing vigorous changes, driven by regulatory requirements for trials in the region. These changes encompass numerous reforms aimed at simplifying procedures and enhancing operational efficiency. Notably, Brazil has enacted significant legislative modifications designed to reduce bureaucratic hurdles and improve the predictability of trial assessments. Such reforms are essential as they facilitate quicker approvals and foster a more favorable atmosphere for health research, effectively addressing the regulatory requirements for Latin America trials.
Looking ahead to 2025, emerging trends indicate a substantial shift towards digitalization within governance structures across the region. This digital transformation is crucial, promising to expedite approval processes and enhance transparency, thereby simplifying the navigation of regulatory requirements for Latin America trials for researchers. For instance, the requirement for research data to be preserved for five years post-registration in Brazil underscores the importance of robust data management practices in this evolving landscape.
Moreover, it is imperative to recognize that requests for new registration by the supporting institution are prohibited within a 12-month period following cancellation, adding another layer of complexity to the framework. Additionally, the recent reforms addressing regulatory requirements for Latin America trials have garnered positive responses from industry stakeholders. As highlighted by Pedro Pablo Kuczynski, former President of Peru, the removal of previous obstacles has unveiled new opportunities for conducting medical studies, particularly in pediatric groups that were previously constrained.
This paradigm shift not only broadens the scope of research but also ensures compliance with regulatory requirements for Latin America trials. A pertinent case study illustrating these advancements is the collaboration between bioaccess™ and Caribbean Health Group, which aspires to establish Barranquilla as a premier destination for clinical studies in Latin America. Supported by Colombia's Minister of Health, this initiative seeks to leverage the region's expertise and insights, ultimately driving economic growth through job creation and healthcare improvement. Bioaccess™ offers a comprehensive suite of services, including feasibility studies, site selection, compliance reviews, experimental setup, and project management.
These capabilities are vital in ensuring that clinical studies are conducted efficiently and in alignment with compliance standards, directly contributing to the overall success of investigative initiatives in the region. Specifically, the emphasis on the Investigator’s Brochure (IB) requirements highlights the sponsor's obligation to provide essential information about the experimental drug, encompassing its formulation, pharmacological effects, and safety data—critical components that guarantee the studies are executed safely and effectively. Furthermore, the import petition must adhere to documentation requirements, including a commercial invoice and research ethics committee approval, underscoring the significance of thorough preparation in meeting the regulatory requirements for Latin America trials.
In summary, the advancements in the regulatory environment, coupled with strategic partnerships such as that of bioaccess and Caribbean Health Group, present substantial opportunities for researchers and Medtech companies to conduct trials with greater efficiency. By facilitating job creation and contributing to economic growth, bioaccess is playing a pivotal role in enhancing medical knowledge and patient care throughout Latin America.
Conclusion
The clinical trials landscape in Latin America is rapidly evolving, presenting a unique blend of opportunities and challenges for researchers and sponsors. The region's diverse regulatory frameworks, particularly Colombia's cost-effective and streamlined processes in contrast to Argentina's stringent requirements, underscore the necessity of comprehending local regulations. Colombia has notably emerged as a prime destination for clinical trials, bolstered by its robust healthcare system, competitive pricing, and supportive government initiatives that promote innovation and R&D.
Good Clinical Practice (GCP) stands as a cornerstone for the successful execution of trials, safeguarding the rights and safety of participants while bolstering the credibility of research findings. As the region adapts to new regulatory trends, including increased digitalization and legislative reforms, stakeholders must prioritize compliance and ethical standards to adeptly navigate this intricate environment.
Furthermore, overcoming recruitment challenges through culturally sensitive strategies and community engagement can significantly enhance participation rates, ultimately resulting in more successful outcomes. The ongoing advancements in regulatory processes, combined with strategic partnerships and a commitment to health equity, position Latin America as a burgeoning hub for clinical research.
In summary, grasping the intricacies of Latin America's clinical trial landscape is vital for unlocking its potential. By leveraging local expertise, adhering to GCP, and embracing the region's dynamic regulatory changes, stakeholders can effectively navigate these waters and contribute to the advancement of medical research and healthcare improvements across the continent.
Frequently Asked Questions
What is the regulatory environment for medical studies in Latin America?
The regulatory environment is shaped by diverse frameworks that differ significantly among countries, each governed by its respective regulatory body. For instance, Brazil's ANVISA and Mexico's COFEPRIS play crucial roles in ensuring compliance with both local and international standards.
What advantages does Colombia offer for first-in-human studies?
Colombia offers several competitive advantages including cost efficiency, regulatory speed, high-quality healthcare, effective patient recruitment, and robust R&D tax incentives. Savings can exceed 30% compared to similar studies in North America or Western Europe.
How long does the review process take in Colombia for medical studies?
The total review process involving the IRB/EC and the Ministry of Health (INVIMA) typically takes only 90-120 days.
How does Colombia's healthcare system rank globally?
The World Health Organization ranks Colombia's healthcare system as #22 globally, with a well-covered patient pool due to approximately 95% of individuals being under universal healthcare.
What tax incentives are available for research and innovation in Colombia?
Significant tax deductions include a 100% tax deduction for eligible projects, a 25% tax discount, a 50% future tax credit, and around $10 million in government grants.
What collaborations are being established to enhance medical studies in Colombia?
Collaborations like that between bioaccess™ and Caribbean Health Group aim to establish Barranquilla as a leading hub for medical studies. Partnerships such as GlobalCare Clinical Trials with bioaccess™ have improved recruitment efficiency significantly.
What recent legislative changes have affected clinical research in Brazil?
Brazil's Law 14.874/24, enacted in 2024, has streamlined the trial approval process, allowing local ethics committees to independently approve protocols, which is expected to reduce approval times significantly.
How do Argentina's regulatory requirements compare to those of Brazil and Colombia?
Argentina maintains a more rigorous oversight environment with comprehensive documentation and longer approval timelines, presenting challenges for sponsors as of 2025.
What is the importance of understanding regulatory requirements in Latin America?
Grasping the regulatory requirements is crucial for organizing research initiatives effectively, as the nuances can greatly impact the success and efficiency of clinical trials.
What is the role of educational initiatives in improving participation in experimental studies?
Targeted educational initiatives are essential to address the knowledge gap among healthcare professionals and patients regarding the benefits of experimental studies, which can enhance participation rates.




