Introduction
In the realm of clinical trials, the meticulous definition of endpoints stands as a cornerstone for evaluating the efficacy of medical interventions. Endpoints, which serve as predefined outcomes, not only shape the objectives of a study but also play a critical role in guiding statistical analyses and interpreting results.
This comprehensive overview delves into the various types of endpoints—including primary, secondary, exploratory, and surrogate—highlighting their significance in clinical research. By understanding the nuances of these classifications, researchers can enhance the clarity and applicability of their findings, ultimately contributing to improved patient outcomes and informed regulatory decisions.
As the landscape of clinical trials continues to evolve, the importance of well-defined endpoints remains paramount in ensuring that research aligns with both clinical relevance and regulatory expectations.
Defining Endpoints in Clinical Trials: A Comprehensive Overview
Endpoints, which are predefined outcomes, play a pivotal role in assessing the effectiveness of clinical interventions. They serve as crucial standards for assessing the effectiveness of a treatment and are divided into two primary groups: primary and secondary measures. A thorough comprehension of these points is essential for researchers, as they not only determine the project's goals but also affect the statistical evaluations and interpretation of the findings.
By clearly specifying objectives, researchers enhance the robustness of their studies, ensuring that the findings are applicable to clinical practice. Typical summary measures for continuous outcomes include metrics such as the average (mean, median) and spread (minimum, maximum, range, quartiles, interquartile range, standard deviation, variance). As highlighted by Ann Yellowlees, a company founder and director of statistics with extensive experience in medical statistics, the careful definition of objectives is crucial within a regulatory environment, as it ensures compliance and facilitates the approval process.
In the context of pragmatic research, detecting certain outcomes may require additional efforts, which can complicate the study's pragmatic nature. Nonetheless, this remains achievable and highlights the significance of clearly defined goals to ensure clarity and relevance in medical research. Ongoing conversations regarding trial guidance have garnered interest, with 20 public remarks stressing the importance of the secondary endpoint clinical trial, underscoring the increasing acknowledgment of their relevance in research.
For example, binary outcomes—defined as results that can be classified into two separate groups, such as reaching a predetermined level of change in a medical measurement—offer clear illustrations of how outcomes can be classified. Examples include dose limiting toxicity and specific changes in hemoglobin levels.
Understanding Secondary Endpoints: Importance and Distinctions
Secondary measures serve as crucial supplementary outcomes that enrich the understanding of a treatment's overall effectiveness. While primary objectives are designed to evaluate the main therapeutic advantage, secondary measures include a wider variety of health outcomes, such as quality of life evaluations, biomarkers, and other pertinent information. This differentiation is crucial for scholars, as secondary measures can greatly improve the clarity of study outcomes and inform subsequent inquiries.
For instance, a medical study might show a significant enhancement in its main goal; however, secondary measures could reveal essential details about safety or tolerability, thereby offering a more thorough overview of the treatment's effect.
Expert opinion emphasizes the necessity of incorporating secondary endpoint clinical trial outcomes into clinical trials. As noted by leading researchers, "regulatory perspectives on multiple endpoints highlight the need for careful consideration of these additional measures." Furthermore, the case analysis titled "Durable Benefit of Rituximab Maintenance Post-Autograft in Follicular Lymphoma" exemplifies this principle.
In this study, researchers tracked patients with relapsed follicular lymphoma to assess the long-term advantages of Rituximab maintenance therapy following autologous stem cell transplantation. The 12-year follow-up results indicated a durable benefit from Rituximab maintenance, significantly improving patient outcomes. This highlights how a secondary endpoint clinical trial can clarify treatment effectiveness and guide medical practice.
Such insights highlight the significance of secondary objectives not only in assessing treatments but also in influencing health policy without inappropriate inference by ensuring that research aligns with predetermined primary outcomes.
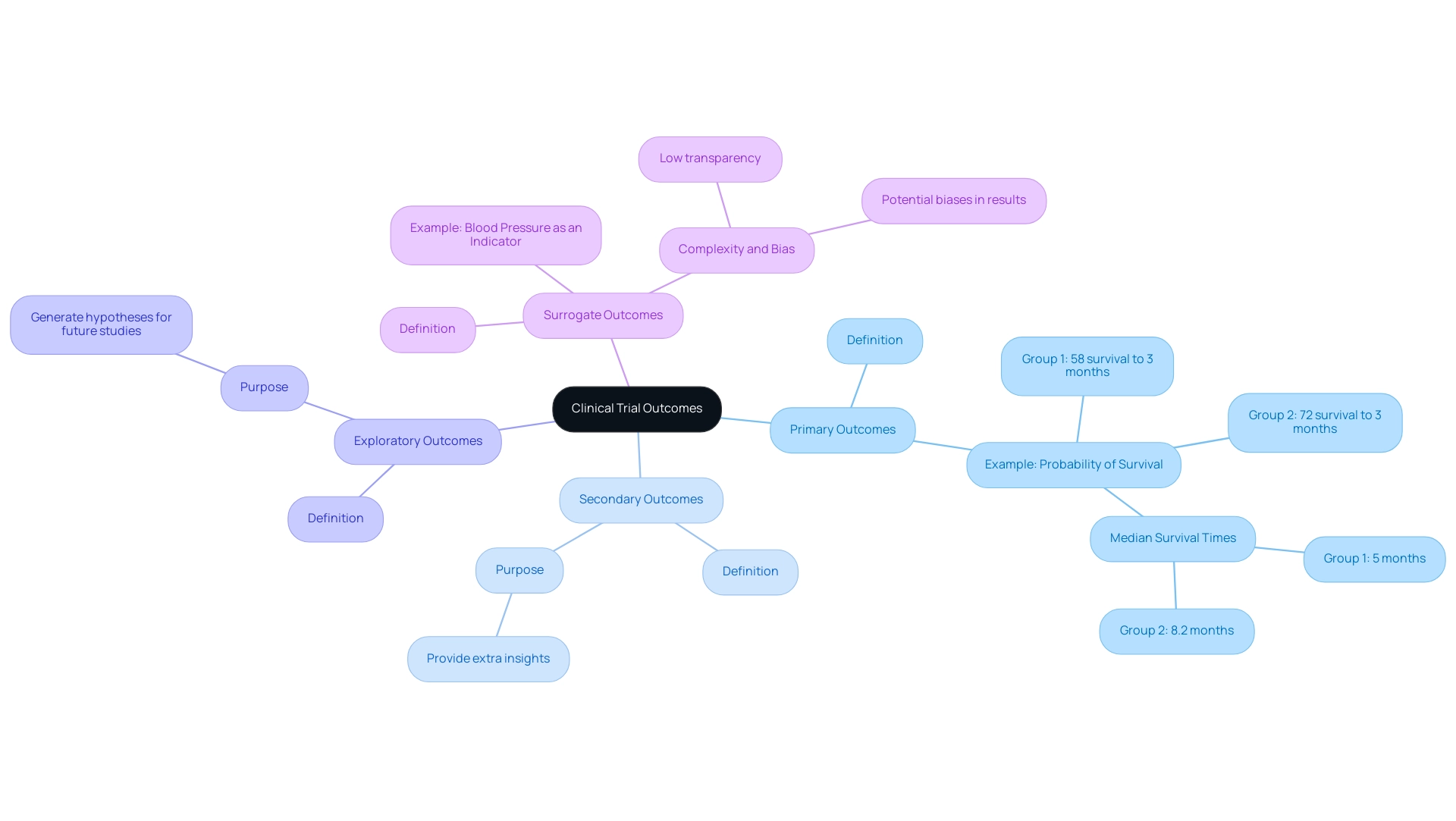
Exploring Different Types of Endpoints: From Primary to Surrogate
Outcomes in clinical trials are primarily classified into four categories: primary, secondary, exploratory, and surrogate outcomes. Primary objectives signify the main results of interest that the research intends to measure, serving as the basis for evaluating the effectiveness of a treatment. For instance, in one treatment group, the probability of survival to 3 months is approximately 58%, while in another group, it is 72%, with median survival times of 5 months and 8.2 months respectively.
In a secondary endpoint clinical trial, secondary measures provide extra insights that can improve the comprehension of treatment effects but are not the primary emphasis of the study. Exploratory objectives are utilized to generate hypotheses for subsequent investigations, providing a broader context for the data collected. In contrast, surrogate outcomes are indirect measures of clinical benefit, such as using blood pressure as an indicator for cardiovascular events.
While these surrogate markers can simplify the assessment of treatment effects, they may introduce complexity, leading to low transparency and potential biases in results. This complexity is especially significant when considering composite measures, which can obscure the true effects of treatments. Comprehending these differences is vital for researchers seeking to choose outcomes that directly correspond with their research goals, thus improving the significance and applicability of research results.
As highlighted by Charlie McLeod,
Such guidelines may be beneficial for end-users and help reduce research waste.
The evolving landscape of selection criteria, as illustrated by the case study titled 'Optimisation of Endpoints for Late Phase Trials,' emphasizes the need for streamlined identification of prioritized outcomes in the context of a secondary endpoint clinical trial, ensuring that these measures truly reflect meaningful benefits for patients.
Regulatory Considerations for Endpoint Selection in Clinical Trials
The choice of targets in clinical trials is governed by stringent regulatory guidelines established by organizations such as the FDA and EMA. Researchers must ensure that their selected goals are not only clinically relevant but also statistically valid, particularly in the context of competing events such as relapse and death, which require specific analytical approaches to accurately interpret time-to-event data. This dual justification is essential for maintaining the integrity of the study and for facilitating the approval process of new therapies.
A notable aspect of selection is its influence on regulatory decisions; for instance, the FDA's recent public feedback on draft guidance highlighted the necessity of carefully considering the secondary endpoint clinical trial measures in the context of primary objectives. This feedback, which sparked significant debate regarding relevance of the endpoints, underscores the importance of proper documentation and rationale in securing regulatory acceptance. Aligning endpoint selection with regulatory expectations not only enhances the credibility of the research but also fosters smoother interactions with regulatory agencies, ultimately impacting approval rates.
Comprehensive research study management services, including:
- feasibility assessments
- site selection
- compliance evaluations
- study setup
- import permits
- project management
- reporting
are essential for navigating these complexities. With expertise from professionals such as Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, researchers can effectively manage these processes. Their roles are pivotal in ensuring that the evaluations adhere to regulatory standards, thus facilitating smoother approvals.
As expressed by Robert Peter Gale, a consultant and advisor in the field, navigating these regulatory complexities is essential for successful research outcomes. Furthermore, the extensive information available on this topic, including a comprehensive PDF file of 1000.6KB, reflects the depth of guidance and resources that researchers must engage with in this complex landscape.
Strategies for Optimizing Endpoint Selection in Clinical Trials
To enhance selection processes in medical trials, researchers should adopt several critical strategies:
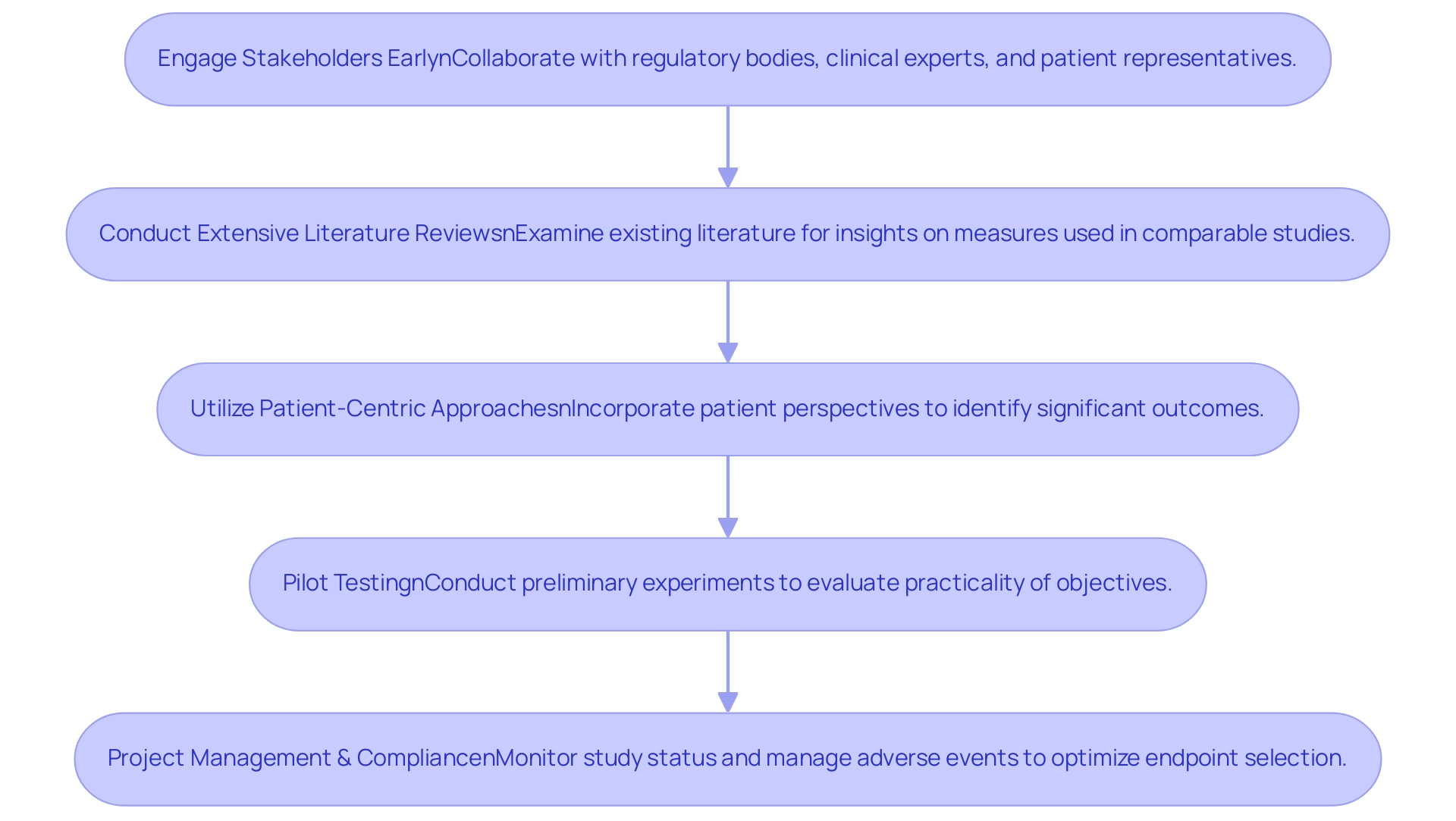
- Engage Stakeholders Early: Actively collaborating with regulatory bodies, clinical experts, and patient representatives is essential to identify targets that resonate with key research questions. Early involvement ensures that the chosen targets are relevant and meaningful to all parties involved.
- Conduct Extensive Literature Reviews: Examining existing literature can uncover frequently utilized measures in comparable studies, providing valuable insights that can assist in the selection process. This approach not only enhances the reliability of selection options but also aligns them with established practices.
- Utilize Patient-Centric Approaches: Incorporating the perspectives of patients is vital in understanding which outcomes hold significance for them. For instance, the EuroQol five-dimensional questionnaire (EQ-5D) serves as a generic quality of life tool that can help in identifying relevant patient-reported outcomes.
By emphasizing such results, researchers can increase the significance and influence of chosen objectives, thereby enhancing study outcomes.
- Pilot Testing: Conducting preliminary experiments enables researchers to evaluate the practicality of chosen objectives prior to the main examination. This step can facilitate necessary adjustments, ensuring that the targets are both practical and aligned with research objectives.
A thorough strategy for clinical trial administration, including feasibility assessments, site selection, compliance evaluations, trial configuration, and initiation processes, is essential for the success of a secondary endpoint clinical trial. The intricacy of selection in this context is emphasized by persistent difficulties encountered in this field, as referenced in the case analysis titled 'Challenges in Selection for Clinical Trials,' which underscores the necessity for streamlined procedures and guidelines.
Furthermore, Amorim and Cai's tutorial on modeling recurrent events in epidemiology offers valuable insights into the statistical considerations that can further enhance outcome selection. Ken Getz emphasizes the urgency of collective investment in stakeholder engagement, stating,
Collective investment into leveraging popular culture and mass media as an educational medium is urgently needed.
Furthermore, robust project management practices are essential in monitoring the study status and managing serious and non-serious adverse events, which directly influence the optimization of endpoint selection.
By effectively implementing these strategies, alongside compliance with regulatory standards such as those set by INVIMA, researchers can enhance the robustness and likelihood of success in their clinical trials.
Conclusion
In the intricate landscape of clinical trials, the meticulous definition and selection of endpoints are fundamental to the success of research endeavors. The classification of endpoints into primary, secondary, exploratory, and surrogate categories not only shapes the objectives of a study but also influences the overall interpretation and applicability of results. By understanding these distinctions, researchers can ensure that their findings are relevant and impactful, ultimately contributing to enhanced patient outcomes and informed regulatory decisions.
The importance of secondary endpoints, in particular, cannot be overstated. They provide supplementary insights that enrich the understanding of treatment efficacy and safety, offering a more holistic view of a clinical intervention's impact. As demonstrated in case studies, such as the enduring benefits of Rituximab maintenance therapy, secondary endpoints can reveal critical information that informs clinical practice and health policy. This underscores the necessity for researchers to incorporate these additional measures into their trials, aligning closely with regulatory expectations to facilitate smoother approvals.
Moreover, the strategies for optimizing endpoint selection include:
- Engaging stakeholders early
- Conducting comprehensive literature reviews
- Utilizing patient-centric approaches
- Implementing pilot testing
These strategies are essential for enhancing the robustness of clinical trials. By prioritizing relevance and practicality, researchers can navigate the complexities of endpoint selection and ensure that their studies yield meaningful and actionable insights. As the field of clinical research continues to evolve, the commitment to well-defined endpoints remains a cornerstone for advancing medical science and improving patient care.
Frequently Asked Questions
What are endpoints in clinical research?
Endpoints are predefined outcomes that play a crucial role in assessing the effectiveness of clinical interventions. They serve as standards for evaluating treatment effectiveness and are divided into primary and secondary measures.
Why is understanding endpoints important for researchers?
A thorough comprehension of endpoints is essential for researchers as they determine the project's goals and affect statistical evaluations and interpretation of findings, enhancing the robustness and applicability of the studies to clinical practice.
What are primary and secondary measures?
Primary measures evaluate the main therapeutic advantage of a treatment, while secondary measures include a wider variety of health outcomes, such as quality of life evaluations and biomarkers, enriching the understanding of a treatment's overall effectiveness.
How do summary measures for continuous outcomes work?
Typical summary measures for continuous outcomes include metrics such as the average (mean, median) and spread (minimum, maximum, range, quartiles, interquartile range, standard deviation, variance).
What role do secondary measures play in clinical trials?
Secondary measures serve as supplementary outcomes that can reveal essential details about safety or tolerability, offering a more thorough overview of the treatment's effect and improving the clarity of study outcomes.
Can you provide an example of secondary endpoints in a clinical trial?
An example is the study titled 'Durable Benefit of Rituximab Maintenance Post-Autograft in Follicular Lymphoma,' which tracked patients to assess the long-term advantages of Rituximab maintenance therapy, highlighting how secondary endpoints can clarify treatment effectiveness.
How do regulatory perspectives influence the use of endpoints in clinical trials?
Regulatory perspectives emphasize the need for careful consideration of multiple endpoints, highlighting their importance in assessing treatments and influencing health policy without inappropriate inference.
What is the significance of clearly defined goals in medical research?
Clearly defined goals ensure clarity and relevance in medical research, making it easier to interpret findings and align research with predetermined primary outcomes.




