Introduction
In Paraguay, the landscape of medical technology is rapidly evolving, fueled by a burgeoning demand for innovative medical devices and the critical role of Contract Research Organizations (CROs). These specialized entities provide essential support to medical device companies, guiding them through the complex clinical research process that is vital for regulatory compliance and market entry.
As the Medtech sector expands, understanding the intricacies of CRO services becomes paramount for stakeholders aiming to navigate the regulatory maze and capitalize on emerging opportunities. This article delves into the current state of Medtech CRO services in Paraguay, highlighting their significance in advancing clinical research, the challenges they face, and the technological innovations that are reshaping their operations.
Through a comprehensive examination of these elements, it becomes clear that Medtech CROs are not just facilitators of clinical trials; they are pivotal players in the advancement of healthcare solutions that ultimately enhance patient outcomes.
Understanding Medtech CRO Services in Paraguay
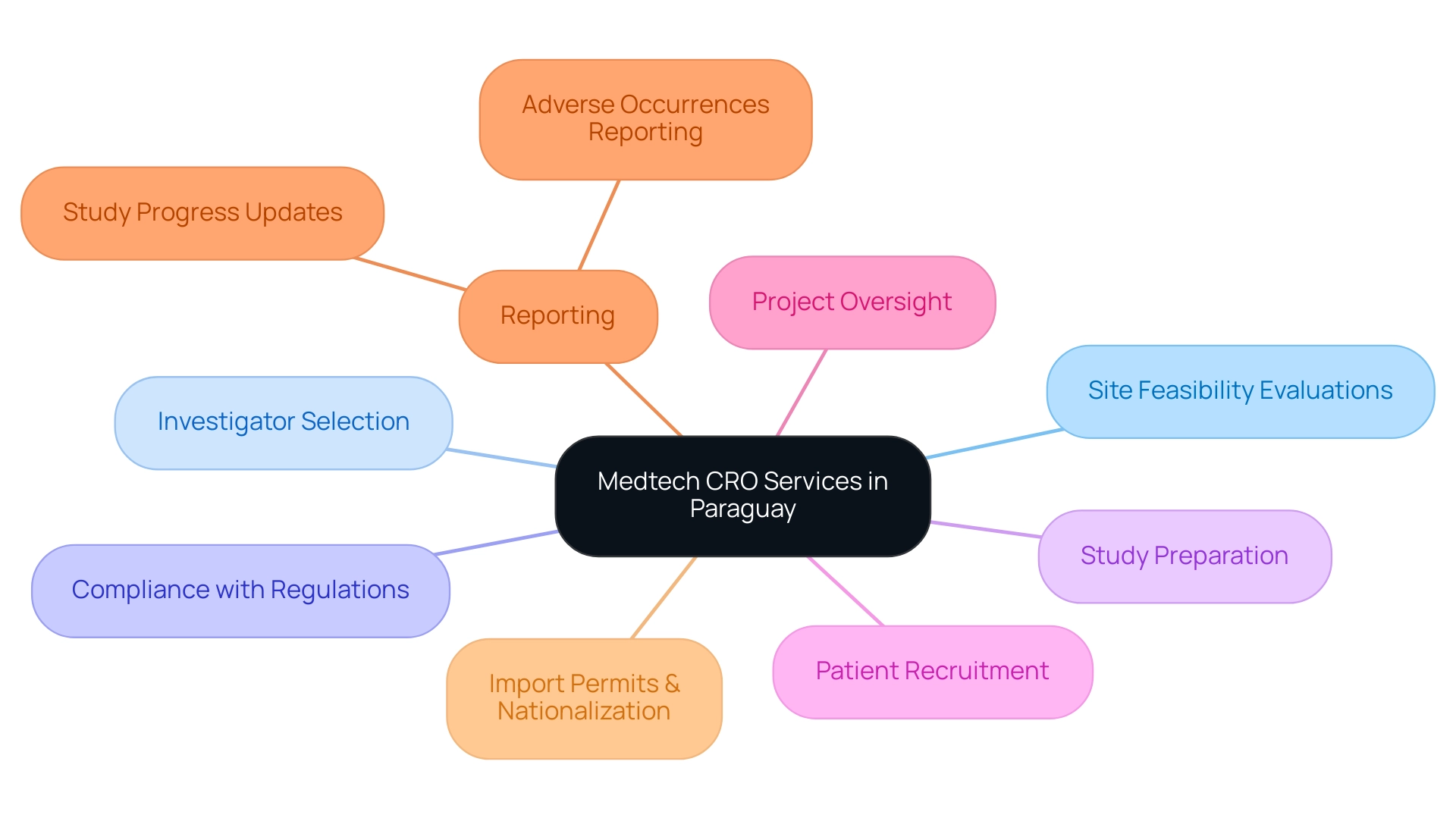
Medtech CRO Services Paraguay involve specialized entities that provide extensive assistance for medical device companies during the research process. These offerings include a broad spectrum of tasks, such as:
- Site feasibility evaluations
- Investigator selection
- Compliance with regulations
- Study preparation
- Patient recruitment
- Project oversight
- Reporting on study progress and adverse occurrences
- Import permits and nationalization of investigational devices
Given the convoluted regulatory requirements and the competitive landscape, Medtech CROs play a critical role in navigating these challenges to ensure that clinical studies comply with both local and international regulations.
They facilitate the efficient collection of data vital for the approval and commercialization of medical technologies. In Paraguay, the expansion of the Medtech sector has resulted in a heightened need for Medtech CRO Services Paraguay, which emphasizes their significance in promoting medical innovation and enhancing patient care, especially for startups facing restricted financial resources and extended participant recruitment challenges.
Reach out to discover how our CRO solutions can assist your medical device studies.
Current Landscape and Future Outlook for Medtech Clinical Research in Paraguay
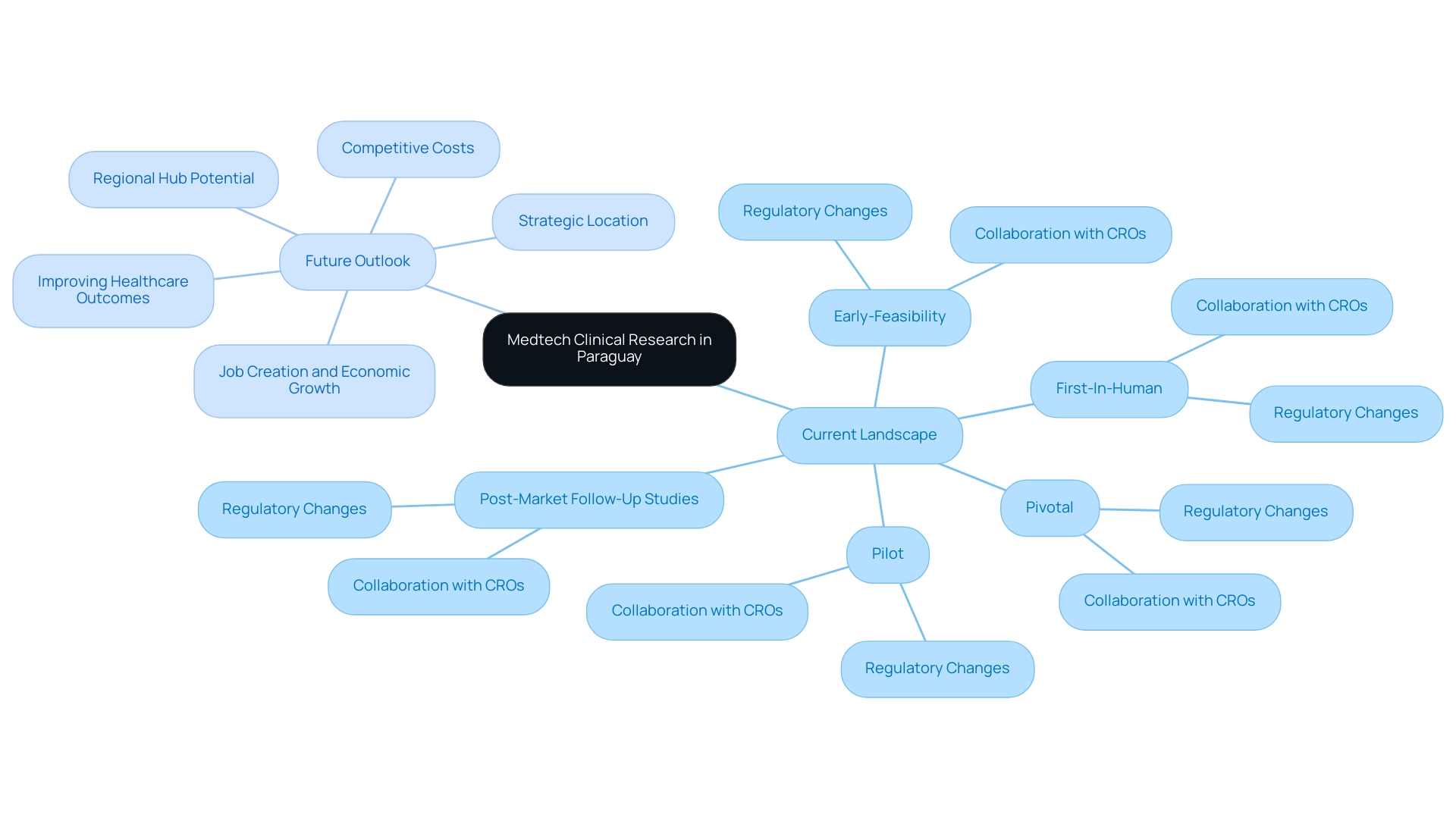
The present environment of Medtech research in Paraguay is marked by an increasing interest from both domestic and global firms, especially as bioaccess® broadens its extensive management offerings in the region. The regulatory framework, overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT), is evolving to accommodate the growing number of research studies being conducted, including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
This evolution includes streamlining approval processes and enhancing collaboration with CROss like bioaccess® to ensure compliance with international standards.
bioaccess®, with more than 20 years of experience in the Medtech sector, is ideally situated to provide specialized offerings such as study setup, compliance evaluations, and project oversight, which are essential for the successful execution of research studies. Looking to the future, Paraguay is poised to become a regional hub for Medtech research, driven by its strategic location, competitive costs, and a commitment to improving healthcare outcomes. As the demand for innovative medical devices continues to rise, the role of Medtech CRO Services Paraguay will be essential in facilitating research and development efforts, ultimately benefiting both the industry and patients alike while promoting job creation and economic growth in the local economy.
Key Services Offered by Medtech CROs
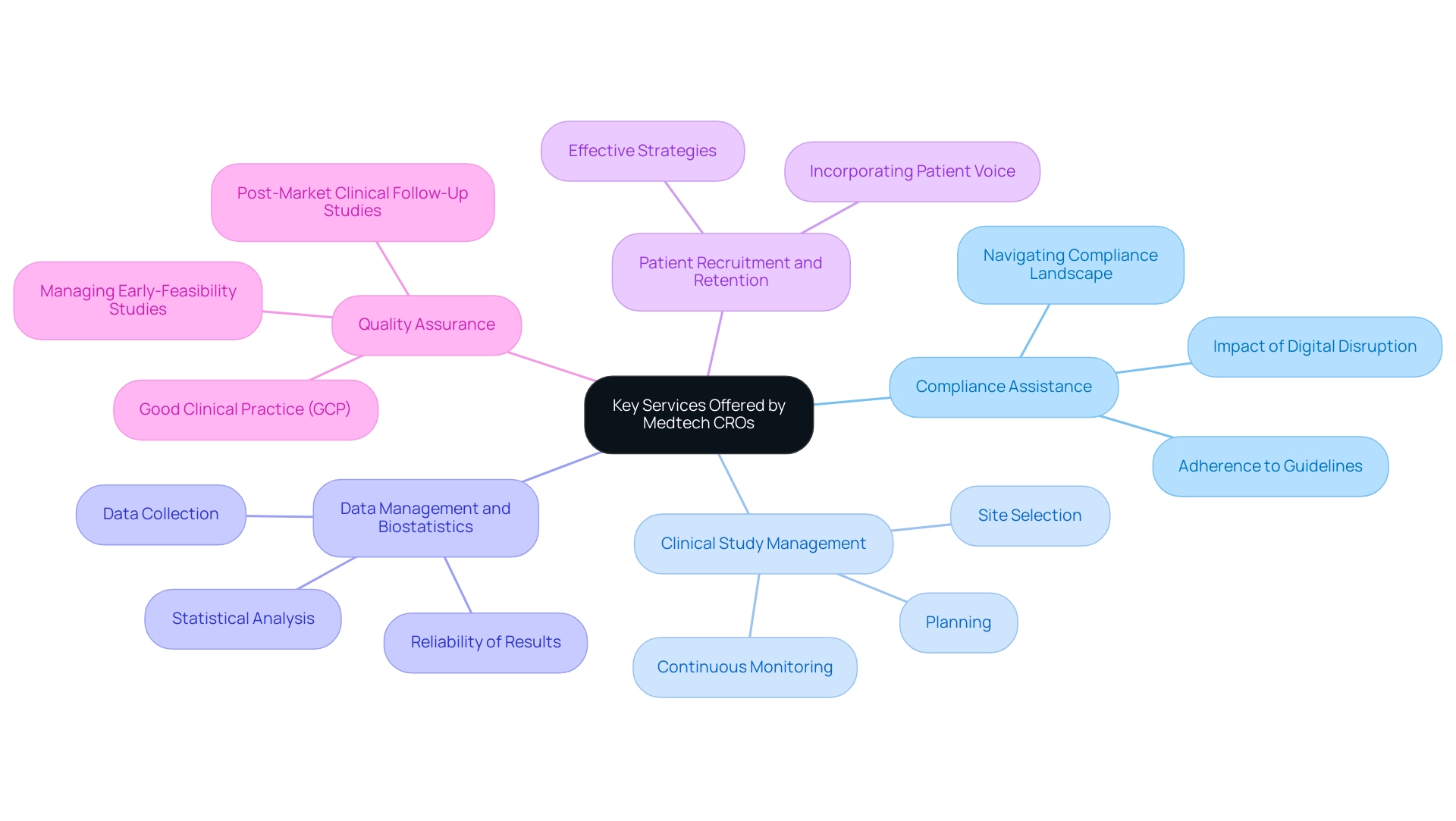
In Paraguay, Medtech CRO Services Paraguay offer a comprehensive range of solutions intended to assist medical device companies throughout the clinical trial process. These services encompass:
-
Expert assistance in navigating the complex compliance landscape ensures adherence to both local and international guidelines.
With more than 20 years of experience in Medtech, our team recognizes the significance of compliance, which is increasingly affected by digital disruption and a greater focus on patient involvement in product development. As emphasized by the European Medicines Agency (EMA), 'The EMA has a well-established process for inclusion of the patient perspective into their decision-making,' highlighting the significance of patient involvement in research.
-
Clinical Study Management: Comprehensive oversight of the entire clinical study process, from initial planning and protocol development to site selection and continuous monitoring. This service is essential for ensuring assessments comply with standards, ultimately acquiring trustworthy data and reducing the need for extra studies. A case study on compliance expectations highlights that following guidance leads to better-structured experiments that produce dependable data, decreasing the likelihood of extra studies being necessary.
-
Data Management and Biostatistics: Providing specialized expertise in data collection, management, and statistical analysis is essential for generating accurate and reliable results. In a rapidly evolving field, the World Economic Forum has identified analytical thinking and complex problem-solving as critical skills for the future of work, especially in this area.
-
Patient Recruitment and Retention: Developing and executing effective strategies to enlist and keep participants is a crucial element in the success of any research study. As highlighted by the EMA, incorporating the patient voice into regulatory decisions improves the relevance and quality of medical research.
-
Quality Assurance: Ensuring that all study aspects comply with Good Clinical Practice (GCP) standards is fundamental to maintaining the integrity of the research and the validity of the outcomes. Additionally, our Medtech CRO Services Paraguay encompass managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Our team has the adaptability and specialized expertise required to manage the intricacies of medical device studies. With Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics, directing our regulatory initiatives, we guarantee that our clients are well-prepared to handle the intricacies of research studies in Latin America. These offerings play an indispensable role in the successful execution of clinical trials, ultimately facilitating the timely introduction of innovative medical devices to the market.
Challenges Faced by Medtech CROs in Paraguay
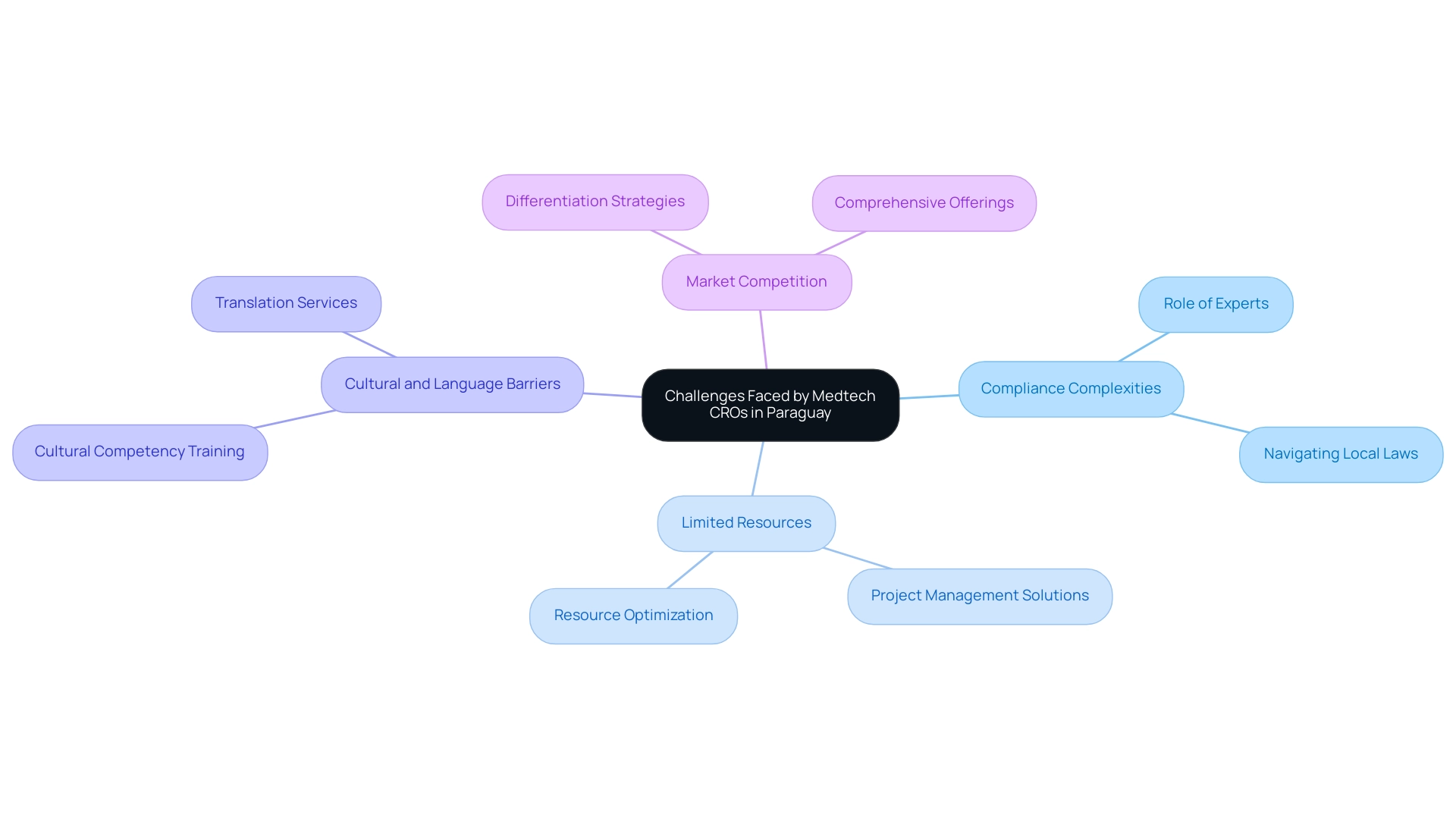
Despite the promising growth of Medtech CRO Services Paraguay, several challenges persist, including:
- Compliance Complexities: Navigating the legal environment can be daunting, particularly for international companies unfamiliar with local laws. Experts like Katherine Ruiz, who has extensive experience in Regulatory Affairs for medical devices and in vitro diagnostics, play a crucial role in guiding compliance and facilitating market entry.
- Limited Resources: Some CROs may struggle with limited resources, impacting their ability to manage multiple projects effectively. Our organization provides comprehensive project management solutions that streamline operations and optimize resource allocation, ensuring that all projects receive the attention they require.
- Cultural and Language Barriers: Collaborating with diverse stakeholders may pose communication challenges, potentially affecting the efficiency of clinical studies. We offer assistance in cultural competency training and translation, which help bridge these gaps and enhance collaboration.
- Market Competition: As the Medtech sector grows, increased competition among CROs can lead to pricing pressures and the need for differentiation. Our comprehensive offerings, including feasibility studies, compliance reviews, setup, and reporting, position us as a leader in the market, enabling us to provide value that distinguishes itself.
Tackling these challenges necessitates strategic planning, investment in training, and nurturing robust relationships with regulatory bodies and stakeholders, all of which are essential to the comprehensive clinical study management offerings, such as Medtech CRO Services Paraguay, provided by our organization.
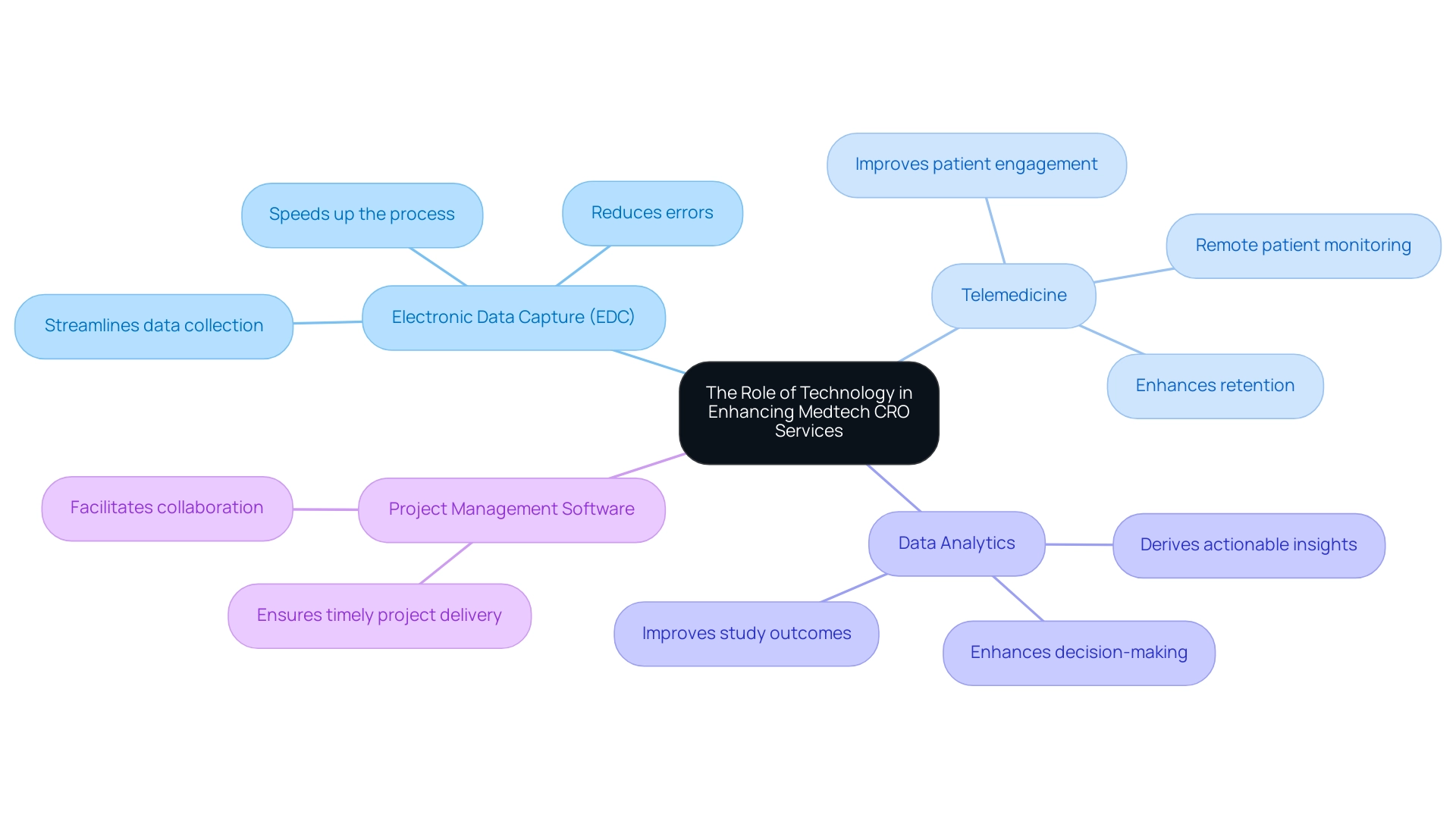
The Role of Technology in Enhancing Medtech CRO Services
Technology plays a pivotal role in enhancing the Medtech CRO Services Paraguay, particularly through the comprehensive offerings of bioaccess®. Key advancements include:
- Electronic Data Capture (EDC): Utilizing EDC systems streamlines data collection, reduces errors, and speeds up the process.
- Telemedicine: The incorporation of telemedicine solutions allows for remote patient monitoring and consultations, improving patient engagement and retention.
- Data Analytics: Advanced analytics tools enable CROss to derive actionable insights from clinical data, enhancing decision-making and study outcomes.
- Project Management Software: Implementing project management tools facilitates better collaboration among team members and stakeholders, ensuring timely project delivery.
Moreover, bioaccess® provides essential support including feasibility and selection of research locations, compliance evaluations, study preparation, import permits, and continuous project oversight. Notably, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and more, with a customized approach designed to meet the unique requirements of each investigation. By leveraging these technological advancements and comprehensive services, Medtech CRO Services Paraguay can significantly improve their operational efficiency and contribute to the successful execution of clinical trials.
Conclusion
The evolving landscape of Medtech CRO services in Paraguay highlights their integral role in advancing medical technology and improving patient outcomes. As detailed throughout the article, these organizations provide essential support across the clinical research process, enabling medical device companies to navigate complex regulatory environments, manage trials effectively, and ensure compliance with both local and international standards.
The current momentum within Paraguay's Medtech sector, fueled by an increasing demand for innovative medical devices, positions CROs as pivotal players in fostering research and development. Their comprehensive suite of services—from regulatory affairs to patient recruitment—addresses critical challenges faced by the industry, including regulatory complexities, resource limitations, and cultural barriers. By strategically leveraging technological advancements, CROs can enhance operational efficiency and streamline clinical trials, ultimately contributing to better healthcare solutions.
Looking ahead, the future of Medtech CRO services in Paraguay appears promising. As the regulatory framework continues to evolve and the demand for medical innovations grows, the collaboration between CROs and medical device companies will become increasingly vital. This partnership not only supports the successful introduction of new technologies but also promotes economic growth and job creation within the local healthcare landscape. The commitment to overcoming challenges and embracing innovation will ensure that Paraguay remains a competitive player in the global Medtech arena, ultimately benefiting patients and healthcare providers alike.
Frequently Asked Questions
What are Medtech CRO Services Paraguay?
Medtech CRO Services Paraguay are specialized services offered by entities that assist medical device companies throughout the research process, including tasks like site feasibility evaluations, investigator selection, compliance with regulations, study preparation, patient recruitment, project oversight, and reporting.
Why are Medtech CROs important in Paraguay?
Medtech CROs are crucial for navigating complex regulatory requirements and the competitive landscape in Paraguay, ensuring that clinical studies comply with local and international regulations, which is essential for the approval and commercialization of medical technologies.
What specific services do Medtech CROs provide?
Medtech CROs provide services such as compliance evaluations, clinical study management, data management and biostatistics, patient recruitment and retention strategies, and quality assurance to maintain Good Clinical Practice (GCP) standards.
What types of studies are conducted under Medtech CRO Services Paraguay?
The types of studies include Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What challenges do Medtech CRO Services face in Paraguay?
Challenges include navigating compliance complexities, limited resources, cultural and language barriers, and increasing market competition among CROs.
How does technology enhance Medtech CRO Services?
Technology enhances these services through advancements like Electronic Data Capture (EDC) for streamlined data collection, telemedicine for remote patient engagement, data analytics for actionable insights, and project management software for improved collaboration.
Who oversees the regulatory framework for Medtech research in Paraguay?
The regulatory framework is overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT), which is evolving to accommodate the growing number of research studies.
What role does bioaccess® play in Medtech CRO Services Paraguay?
Bioaccess® provides over 20 years of experience in the Medtech sector, offering specialized services such as study setup, compliance evaluations, and project oversight, which are essential for executing research studies successfully.

