Overview
Regional research opportunities in Mexico are becoming increasingly appealing to clinical researchers, driven by the country's strategic advantages. These advantages include:
- A diverse patient population
- Cost-effective trial management
- A supportive regulatory environment
Notably, Mexico's advancements in clinical research, particularly within therapeutic areas such as oncology and cardiology, are significant. Furthermore, government reforms aimed at streamlining approval processes position Mexico as a burgeoning hub for innovative medical studies. This landscape not only fosters growth but also invites collaboration among stakeholders in the field.
Introduction
In recent years, Mexico has emerged as a formidable player in the global clinical research landscape, attracting attention for its unique advantages and robust capabilities.
With its strategic geographic location, diverse patient population, and a growing network of experienced clinical research organizations, the country has positioned itself as a prime destination for clinical trials.
The Mexican government's commitment to regulatory reforms and ethical standards has further enhanced its appeal, making it a hotspot for pharmaceutical and medical device companies seeking to conduct innovative studies.
As the landscape continues to evolve, Mexico's role in advancing medical technology and improving healthcare outcomes is set to expand, offering exciting opportunities for researchers and sponsors alike.
The Emergence of Mexico as a Clinical Research Hub
The country has solidly positioned itself as a key participant in the worldwide medical study field. Over the past decade, the nation has experienced a significant rise in research studies, propelled by its strategic geographic position, a diverse and accessible patient demographic, and a growing network of skilled research organizations (CROs). In 2023, the nation accounted for 0.4% of the global research market, with Phase III studies representing 53.2% of the revenue share, underscoring the country's capacity for large-scale investigations.
The government has proactively implemented regulatory reforms designed to expedite the approval process for research, enhancing the country's appeal to pharmaceutical and medical device firms. This encouraging regulatory landscape, coupled with a commitment to ethical standards and cultural sensitivity, has been essential for the success of global companies conducting research in the country. Consequently, the nation is progressively recognized as a center for groundbreaking medical studies, particularly in therapeutic fields such as oncology, cardiology, and diabetes.
The anticipated expansion of Phase I studies, forecasted to be the most profitable and rapidly growing sector, further highlights the dynamic characteristics of regional research opportunities in Mexico. According to the case study 'Market Segmentation Insights,' this segmentation indicates where companies may focus their efforts for growth. This evolution positions the nation as an appealing location for medical researchers interested in regional research opportunities that leverage its unique advantages.
In this context, bioaccess® distinguishes itself with its comprehensive study management services, focusing on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate the intricacies of research studies, ensuring adherence and effective project management. The impact of these medical studies extends beyond research itself, contributing to job creation, economic growth, and healthcare enhancement in the region.
As Dr. Horst Stipp, EVP of Research & Innovation at the Advertising Research Foundation, aptly noted, 'I think of Statista as Google for researchers,' emphasizing the significance of data-driven insights in navigating this evolving market. For individuals seeking to conduct research studies in the country, collaborating with bioaccess® can provide the expertise and support necessary to achieve favorable outcomes.
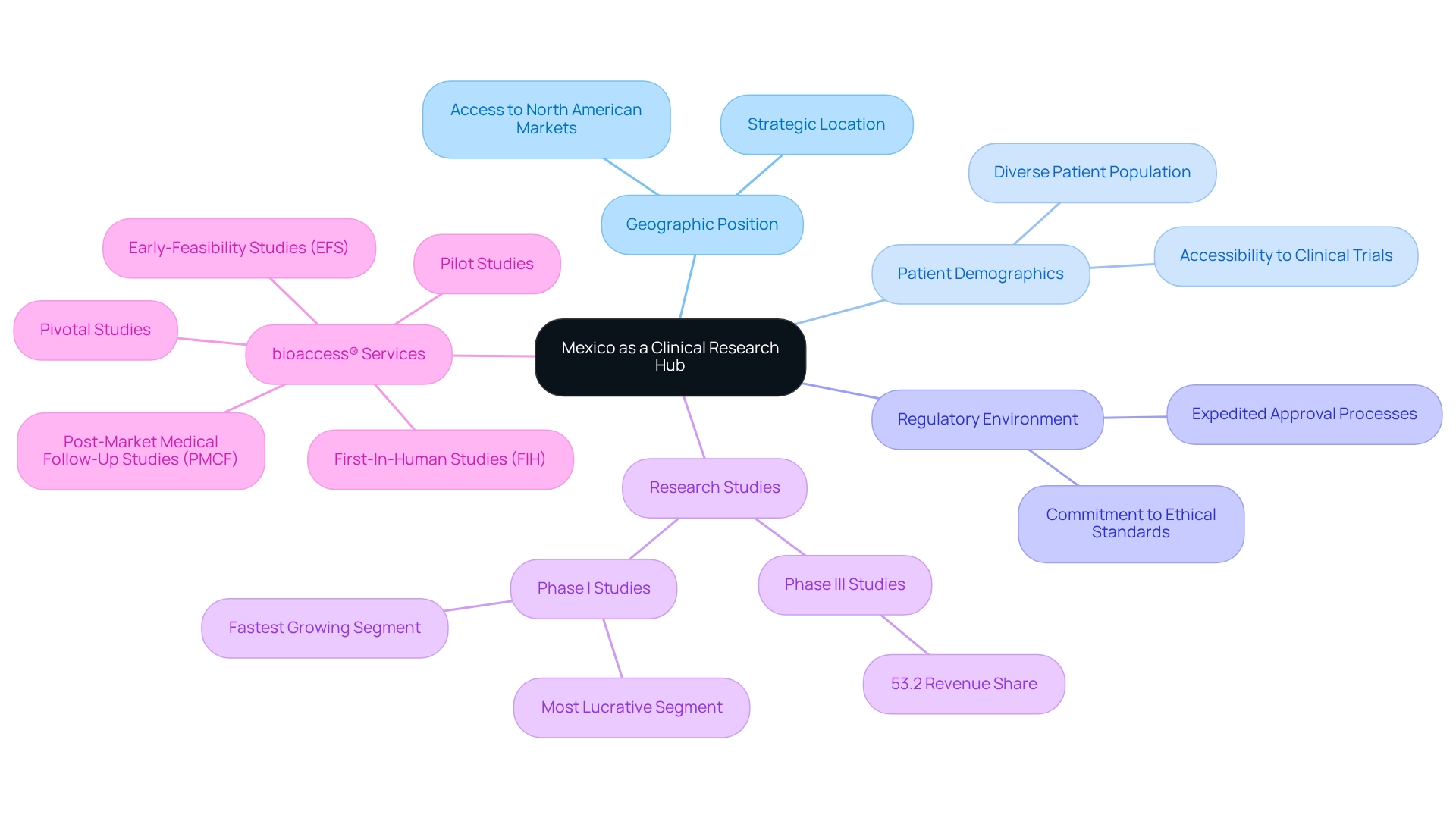
Navigating Mexico's Regulatory Landscape for Clinical Trials
The regulatory environment for medical studies in Mexico is primarily governed by the General Health Law (GHL) and the Secondary Regulations on Medical Research (SRCR). The Federal Commission for Protection against Sanitary Risks (COFEPRIS) serves as the regulatory authority responsible for overseeing research studies. Researchers are required to submit comprehensive protocols for approval, which encompass ethical considerations, informed consent processes, and safety monitoring plans.
Recent reforms have been implemented to streamline the approval process, significantly reducing the time required to initiate experiments. Nevertheless, researchers must remain vigilant regarding compliance with both local and international regulations to uphold the integrity of their studies. At bioaccess®, we emphasize the importance of information security and client trust throughout the research process.
Our grievance and data protection procedures are meticulously designed to address client concerns related to compliance and transparency, thereby reinforcing our commitment to safeguarding sensitive information. Should you have any queries or concerns regarding the processing of your information, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com.
As a leading contract research organization, bioaccess® is dedicated to supporting medical device studies in Latin America, with a particular focus on regional research opportunities in Mexico, aimed at addressing the unique challenges and opportunities present in this dynamic market.

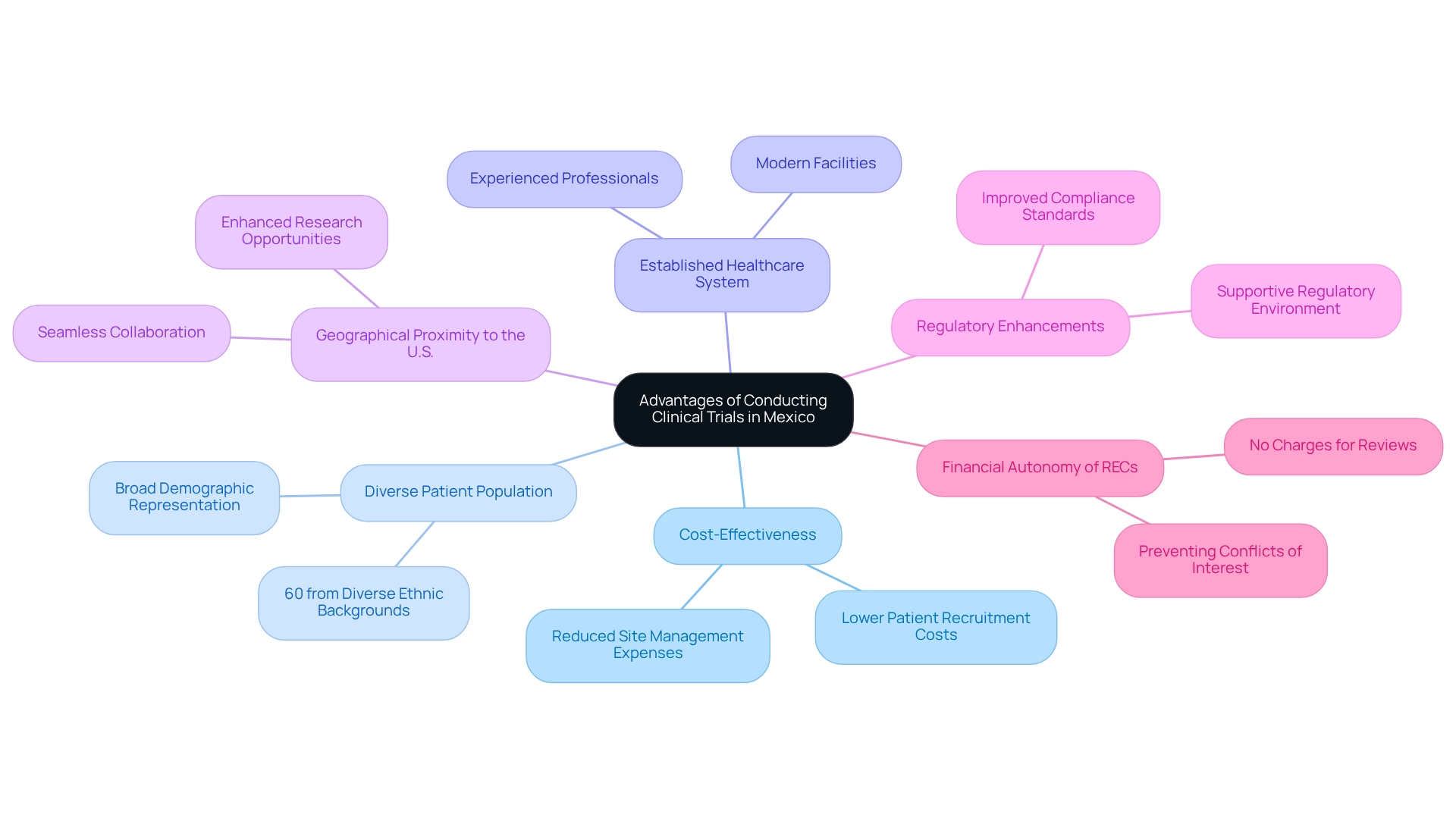
Advantages of Conducting Clinical Trials in Mexico
Carrying out regional research opportunities in Mexico presents numerous advantages, making it an increasingly attractive location for sponsors. The cost-effectiveness of clinical trials in this country is particularly noteworthy, as expenses related to patient recruitment and site management are significantly lower compared to many other regions. This financial efficiency allows sponsors to optimize their budgets for studies while upholding high-quality standards.
Furthermore, Mexico boasts a large and diverse patient population, which is ideal for regional research opportunities that require broad demographic representation. The ability to enlist participants from various ethnic groups enhances the generalizability of medical study results. In 2025, statistics indicate that over 60% of research participants in the country come from diverse ethnic backgrounds, underscoring Mexico's commitment to inclusive research practices.
The well-established healthcare system in Mexico further bolsters the efficient execution of medical studies. Facilities are equipped with modern technology and staffed by experienced professionals, ensuring compliance with international standards. Additionally, the geographical proximity to the United States fosters seamless collaboration between researchers and sponsors, thereby enhancing regional research opportunities and improving the overall effectiveness of the research process.
As emphasized by Julio G. Martinez-Clark, CEO of bioaccess Mexico, 'Overall, Mexico's regulatory enhancements, cost benefits, patient accessibility, and increasing expertise make it an attractive location for regional research opportunities in Mexico.' bioaccess® specializes in comprehensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, this strategic advantage not only accelerates the research timeline but also contributes to the successful advancement of medical technologies in the global market.
Moreover, the financial autonomy of Research Ethics Committees (RECs) in Mexico ensures impartial assessments and transparency in the research process. These committees do not impose charges on sponsors for their reviews and are funded by the health institutions where they operate, effectively preventing conflicts of interest. Additionally, the E-Reporting Industry platform is utilized for managing individual case safety reports at the national level, highlighting the robust safety reporting mechanisms in place.
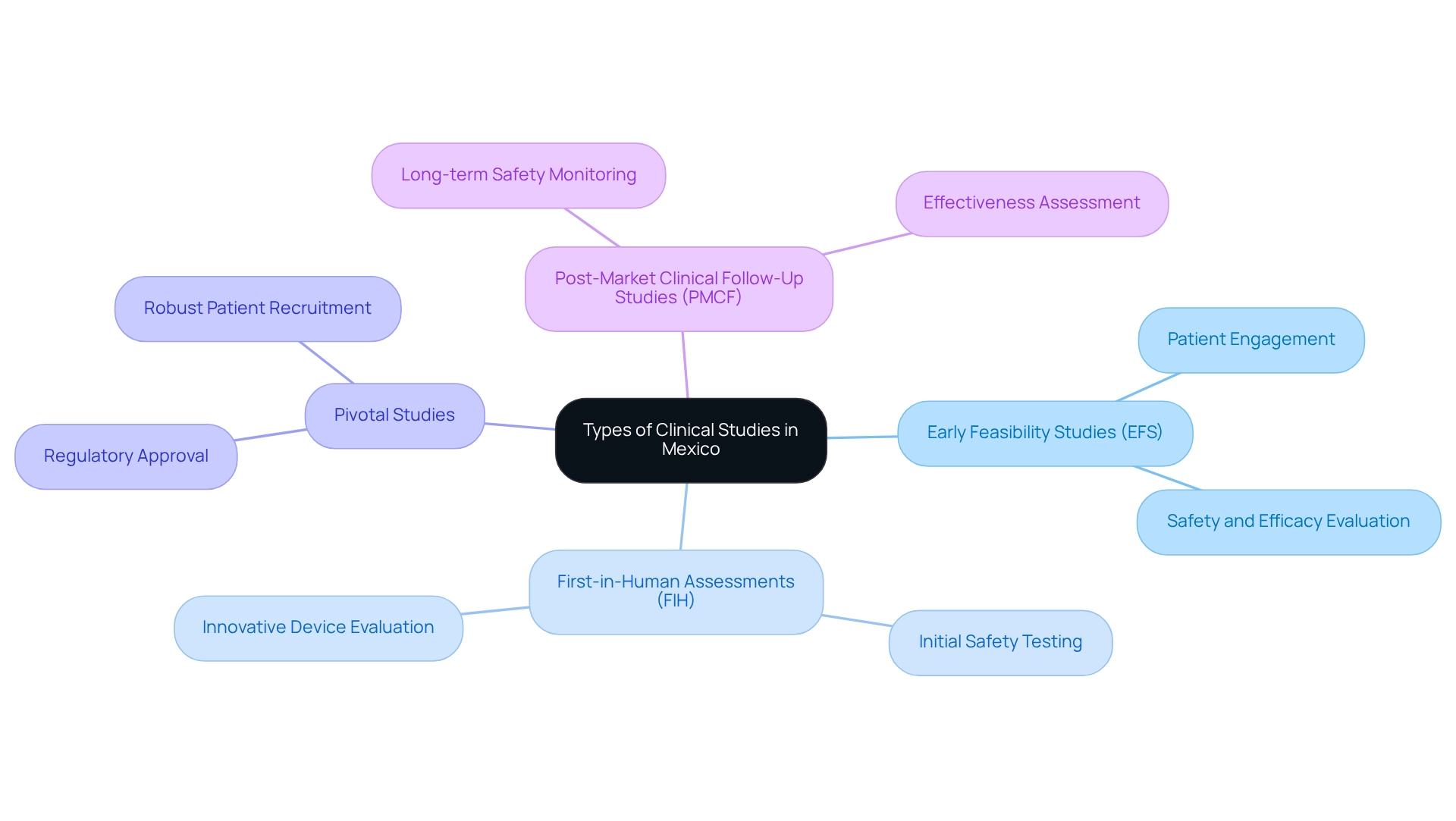
Types of Clinical Studies Available in Mexico
This country stands as a vibrant center for a wide range of clinical studies, including early feasibility studies (EFS), first-in-human (FIH) assessments, pivotal studies, and post-market clinical follow-up studies (PMCF). Early feasibility studies are particularly advantageous for Medtech startups, enabling them to evaluate the safety and efficacy of innovative devices before progressing to larger-scale trials. The success rates of EFS in the country have been notable, with many studies showcasing a high level of patient engagement and recruitment efficiency, critical for timely results.
Pivotal studies, essential for obtaining regulatory approval, can also be conducted effectively here. The country boasts robust patient recruitment capabilities, facilitating the swift execution of these studies. As we look to 2025, the environment of medical investigation in the country continues to evolve, with an increasing number of pivotal studies yielding successful outcomes that contribute to the advancement of medical technology.
Moreover, post-market studies play a vital role in monitoring the long-term safety and effectiveness of medical devices once they reach the market. These studies are crucial for ensuring that devices continue to meet safety standards and provide intended benefits to patients. As the regulatory landscape in Latin America becomes more unified, particularly with the Pacific Alliance focused on streamlining regulatory procedures for medical devices, there are growing regional research opportunities in Mexico, making it an appealing location for Medtech firms seeking to innovate and expand.
bioaccess® possesses the knowledge and tailored strategy required to navigate these research phases successfully, leveraging over 20 years of experience in Medtech. Their extensive research study management services encompass feasibility assessments, site selection, compliance evaluations, study preparation, import permits, project oversight, and reporting, ensuring that each investigation is executed efficiently and effectively.
The worldwide effect of sugar-sweetened drinks (SSBs) on health is considerable, with an estimated 340,000 deaths linked to type 2 diabetes (T2D) and cardiovascular disease (CVD) together in 2020. This statistic underscores the significance of medical research in addressing chronic illnesses prevalent in this country. While Colombia and Paraguay are emerging as pioneers in initial feasibility studies and first-in-human trials in Latin America, the nation's capabilities in executing these studies remain robust and competitive.
As emphasized by Cudhea, F., the burdens of type 2 diabetes and cardiovascular disease associated with SSBs are significant concerns that medical investigation can help alleviate. By concentrating on these health challenges, the nation's medical landscape is not only advancing technology but also contributing to public health enhancements.
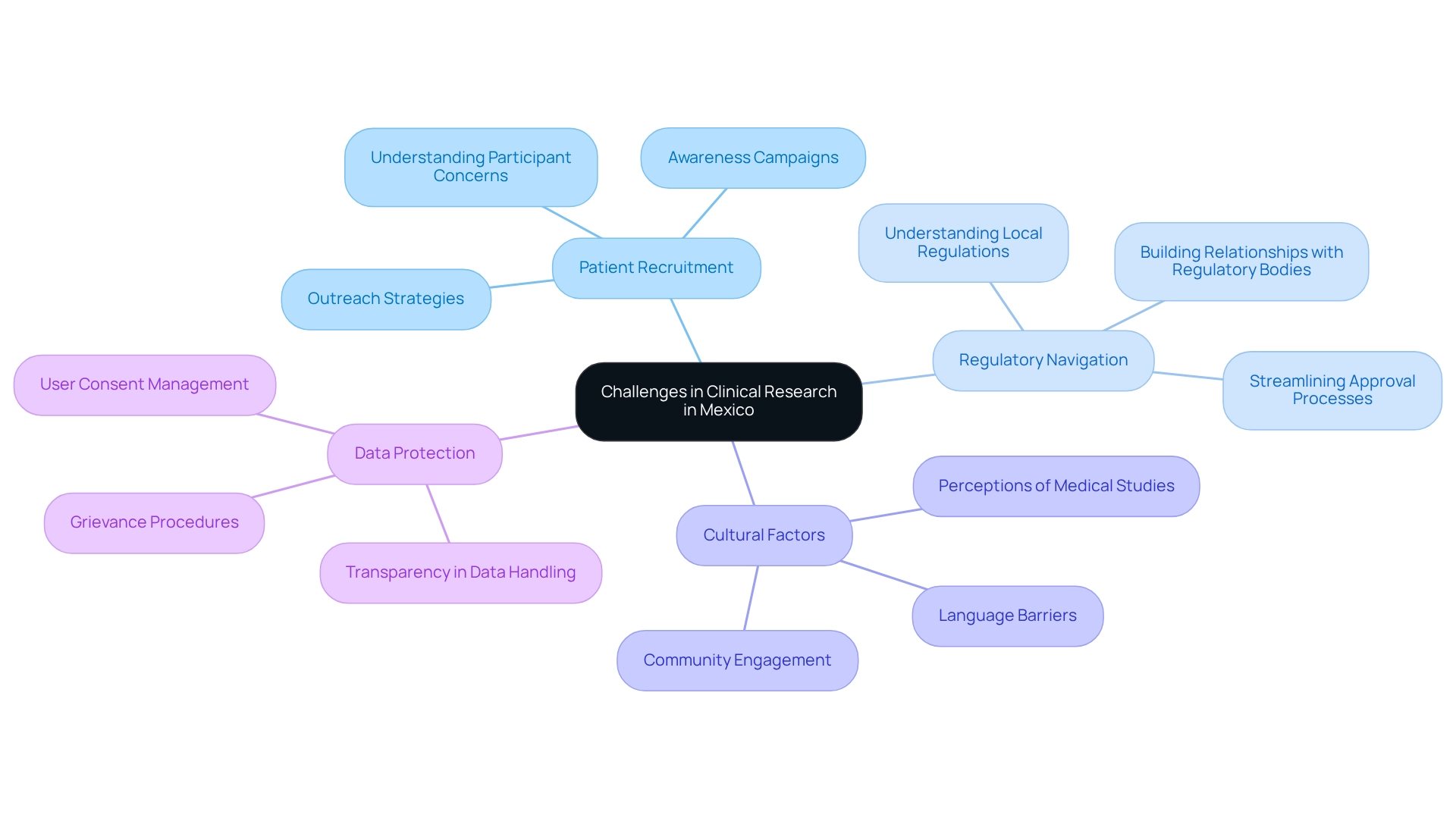
Challenges in Conducting Clinical Research in Mexico
Regional research opportunities in Mexico present a distinct array of challenges, despite their many benefits. One of the primary obstacles is patient recruitment, which can be significantly influenced by a general lack of awareness about research studies among potential participants. To address this, researchers must implement focused outreach strategies that effectively convey the advantages and significance of involvement in research studies.
As Bill Gates aptly noted, "Your most unhappy customers are your greatest source of learning," emphasizing the necessity to understand participant concerns to enhance recruitment strategies.
Navigating the regulatory landscape is another complex aspect, as requirements can vary widely across different states. This necessitates a thorough understanding of local regulations and the establishment of robust relationships with regulatory bodies to ensure compliance and streamline the approval process. The economic landscape in Mexico has been favorably impacted by the North American Free Trade Agreement (NAFTA), signed in 1993, which has increased foreign pharmaceutical investment and led to regional research opportunities in Mexico for medical studies.
Cultural factors play a crucial role in participant engagement and retention. Language barriers and varying perceptions of medical studies can create obstacles that impede recruitment efforts. It is essential for researchers to engage with local communities and stakeholders to foster trust and understanding, thereby enhancing participation rates.
As George R. Bach states, demonstrating care in customer interactions makes the experience special, reinforcing the importance of building trust with potential participants in the recruitment process.
Addressing these complex challenges necessitates careful planning and collaboration with local stakeholders, ensuring that health-related initiatives are not only compliant but also culturally sensitive and effectively communicated to potential participants. bioaccess®, as a leading Contract Research Organization, exemplifies how to navigate these challenges while ensuring information security and client trust. Their commitment to data protection is evident in their grievance procedures, which allow clients to address concerns transparently and in compliance with applicable laws.
For any queries or concerns regarding data protection, clients can contact the Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. Additionally, bioaccess® emphasizes the importance of user consent and cookie management in their operations, further reinforcing their dedication to safeguarding client information. This method not only improves participant confidence but also strengthens bioaccess®'s standing as a dependable ally in the Mexican medical study environment.
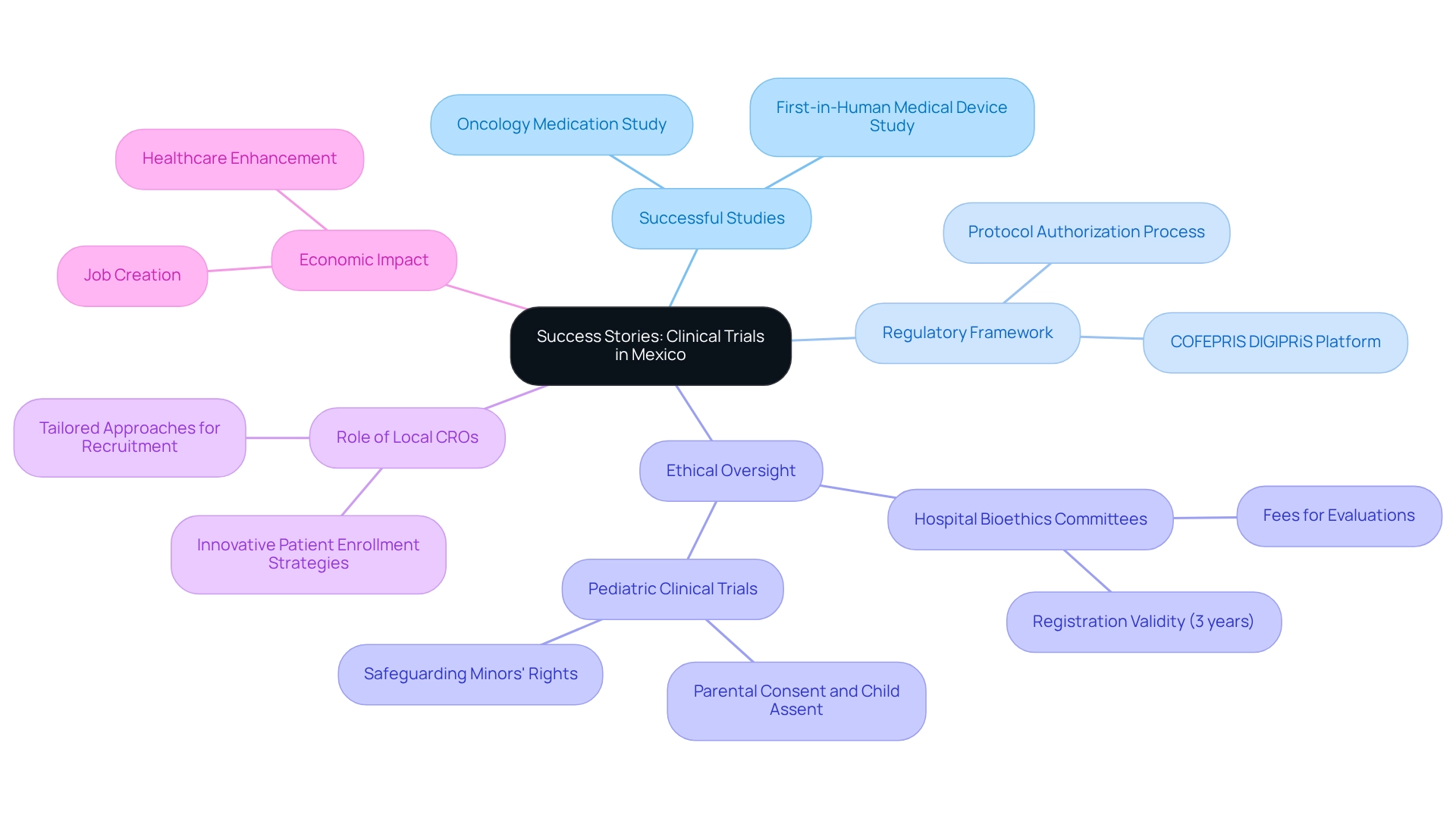
Success Stories: Clinical Trials in Mexico
The nation has emerged as a significant center for investigation, particularly due to the regional research opportunities in Mexico, as evidenced by numerous successful medical studies that underscore its potential. A prime example is a crucial study for a new oncology medication, completed in record time thanks to the innovative patient enrollment strategies employed by local contract development organizations (CROs). These strategies not only streamlined the recruitment process but also ensured a diverse participant pool, thereby enhancing the overall effectiveness of regional research opportunities in Mexico.
The regulatory framework supporting medical experiments in the country is robust, with the application process for protocol authorization facilitated electronically via COFEPRIS’s DIGIPRiS platform. This efficiency is complemented by stable ethical oversight, as the registration for Hospital Bioethics Committees is valid for three years, ensuring consistent ethical review processes. In another instance, a first-in-human study for a groundbreaking medical device, managed by bioaccess®, yielded promising results, subsequently leading to expedited regulatory approval. This achievement highlights the capability of local groups to manage the intricacies of medical studies while upholding high-quality standards.
It is also crucial to recognize that ethics committees may impose fair charges for their evaluations, introducing a financial aspect for sponsors conducting studies in the area. The influence of local CROs on the success of regional research opportunities in Mexico cannot be overstated. Their deep understanding of the regulatory landscape and patient demographics allows for tailored approaches that significantly improve recruitment and retention rates. As a result, these organizations, including bioaccess®, are pivotal in advancing medical innovations and facilitating timely access to new therapies for patients.
With more than 20 years of experience in Medtech, bioaccess® offers unmatched expertise, guaranteeing that studies are performed efficiently and effectively. These success stories not only showcase the efficiency of conducting clinical studies in Mexico but also highlight the regional research opportunities available, demonstrating the increasing confidence in the region's ability to deliver high-quality outcomes in the Medtech field. As Etienne Nichols emphasizes, the significance of efficient screening methods, such as those for cervical cancer, is essential in oncology trials, further underscoring the necessity for ethical considerations in studies. Moreover, the case study on Pediatric Clinical Trials illustrates the ethical obligations for conducting studies involving minors, highlighting the importance of safeguarding their rights and ensuring informed consent throughout the process.
Furthermore, the economic impact of medical studies, including job creation and healthcare enhancement, reinforces the significance of bioaccess's role in the local economy.
Future Trends and Opportunities in Mexican Clinical Research
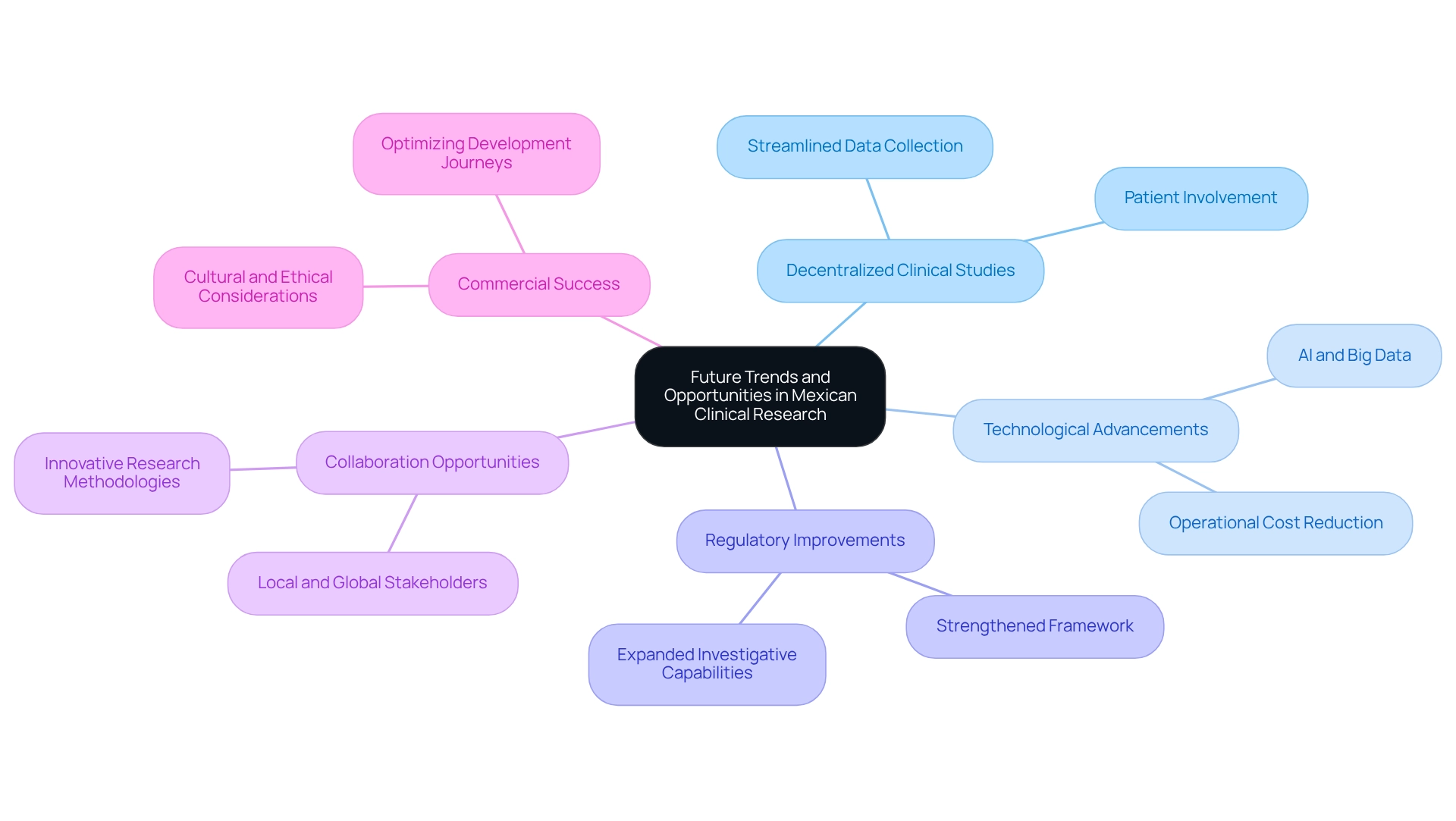
The future of medical research in Mexico is poised for significant transformation, driven by emerging trends and regional research opportunities. A notable shift towards decentralized clinical studies (DCTs) is anticipated, which will enhance patient involvement and streamline data collection processes, thereby making studies more accessible and efficient. By 2025, study sponsors are expected to implement a core outcome set across various study designs, standardizing methodologies and improving patient outcomes through consistent assessment of critical endpoints.
Technological advancements, particularly in artificial intelligence and big data analytics, are set to revolutionize efficiency in testing and reduce operational costs. These innovations will empower researchers to swiftly analyze vast data sets, leading to more informed decision-making and expedited trial outcomes.
As the country strengthens its regulatory framework and expands its investigative capabilities, the environment for trials will become increasingly collaborative. Local and global stakeholders are anticipated to uncover new collaboration opportunities, reinforcing Mexico's role as a significant player in the global medical research arena. For instance, the successful implementation of decentralized medical studies in Mexico has already demonstrated enhanced patient recruitment and retention, showcasing the nation's readiness to embrace innovative research methodologies.
bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) positions them as a leader in the Medtech sector, driving global health improvement through international collaboration and innovation.
Expert insights indicate that the focus on optimizing development journeys will not only target approval-enabling endpoints but also emphasize commercial success. Max Baumann, Head of Execution at Treehill Partners, highlights this necessity, stating, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." This strategic shift is essential as the biotech sector grapples with fundamental business model challenges in a competitive market, underscoring the need for early-stage developers to align their strategies with commercial outcomes.
Furthermore, the dynamics of medical studies can be illustrated by the case study of an Indian biotech firm that initiated a Phase IIb study, highlighting both advancements and challenges faced during the research. This example enhances the understanding of the complexities involved in medical studies.
Lastly, the success of international pharmaceutical firms conducting trials in Mexico relies heavily on their awareness of cultural and ethical considerations. Addressing these factors is crucial for navigating the medical research landscape effectively. Overall, the evolving clinical research landscape in Mexico presents valuable opportunities for those willing to adapt and innovate.
Conclusion
The emergence of Mexico as a leading hub for clinical research is underscored by its strategic advantages, including a diverse patient population, cost-effectiveness, and a supportive regulatory environment. Over recent years, the country has seen a significant increase in clinical trials, particularly in therapeutic areas such as oncology and cardiology. The commitment of the Mexican government to regulatory reforms has streamlined the approval process, making it an attractive location for pharmaceutical and medical device companies.
Despite these advantages, challenges such as patient recruitment and navigating local regulations persist. However, effective outreach strategies and a thorough understanding of the regulatory landscape can mitigate these issues. Furthermore, the presence of experienced contract research organizations like bioaccess® enhances the ability to conduct high-quality studies while ensuring compliance with ethical standards.
Looking ahead, the future of clinical research in Mexico is bright, with trends such as decentralized clinical trials and advancements in technology poised to revolutionize the industry. As collaboration between local and international stakeholders increases, Mexico is expected to solidify its position in the global clinical research arena. This evolving landscape not only promises to advance medical technology but also significantly contributes to improved healthcare outcomes and economic growth in the region. The potential for innovation and success in clinical trials in Mexico makes it an exciting destination for researchers and sponsors alike.
Frequently Asked Questions
What factors contribute to Mexico's emergence as a key participant in global medical research?
Mexico's strategic geographic position, diverse and accessible patient demographic, and a growing network of skilled research organizations (CROs) have significantly contributed to its emergence in the global medical research field.
What percentage of the global research market did Mexico account for in 2023?
In 2023, Mexico accounted for 0.4% of the global research market.
What type of studies represented the majority of revenue in Mexico's research market?
Phase III studies represented 53.2% of the revenue share in Mexico's research market.
How has the Mexican government supported medical research?
The government has implemented regulatory reforms to expedite the approval process for research, enhancing the country's appeal to pharmaceutical and medical device firms.
What therapeutic fields are Mexico recognized for in medical research?
Mexico is progressively recognized for groundbreaking medical studies in therapeutic fields such as oncology, cardiology, and diabetes.
What is the anticipated growth area in Mexico's medical research sector?
Phase I studies are forecasted to be the most profitable and rapidly growing sector in Mexico's medical research.
What services does bioaccess® offer in the realm of medical research?
bioaccess® offers comprehensive study management services, focusing on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF).
What is the role of the Federal Commission for Protection against Sanitary Risks (COFEPRIS) in Mexico's medical research?
COFEPRIS is the regulatory authority responsible for overseeing research studies in Mexico, ensuring compliance with health regulations.
What recent changes have been made to the approval process for research studies in Mexico?
Recent reforms have streamlined the approval process, significantly reducing the time required to initiate experiments.
How does bioaccess® ensure compliance and data protection during research studies?
bioaccess® emphasizes information security and client trust, with grievance and data protection procedures designed to address client concerns related to compliance and transparency.
Where can individuals contact bioaccess® for inquiries regarding their services?
Individuals can contact bioaccess® at IMH ASSETS CORP, located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com.




