Overview
The largest clinical research organizations (CROs) play a vital role in clinical trials by providing essential services such as regulatory compliance, patient recruitment, and data management, which streamline the drug development process. The article emphasizes that CROs like bioaccess® and others are crucial for enhancing research efficiency and patient outcomes, particularly in regions like Latin America, where they leverage local advantages to expedite clinical studies and improve healthcare delivery.
Introduction
In the dynamic world of clinical research, Clinical Research Organizations (CROs) play a pivotal role in bridging the gap between innovative medical advancements and their successful implementation in the healthcare landscape. As pharmaceutical companies increasingly rely on these specialized entities to navigate the complexities of clinical trials, the importance of CROs has never been more pronounced.
From regulatory compliance and patient recruitment to data management and trial execution, these organizations not only expedite the development of new therapies but also enhance patient outcomes. With the industry experiencing rapid growth, particularly in regions like Latin America, the landscape of CROs is evolving, driven by technological advancements and a patient-centric approach.
This article delves into the multifaceted roles of CROs, the challenges they face, and the immense opportunities that lie ahead in the ever-evolving medtech sector.
Defining Clinical Research Organizations (CROs) and Their Importance
The largest clinical research organization plays a crucial role by offering a diverse range of services to assist in the planning, execution, and management of medical studies. These organizations are pivotal in the drug development process, offering specialized knowledge in areas such as regulatory compliance, patient recruitment, and data management. By delegating research studies to CROs like bioaccess®, pharmaceutical firms can access this expertise, which not only speeds up the creation of new therapies but also improves patient outcomes.
Bioaccess® stands out in Latin America, offering comprehensive services such as:
- Feasibility assessments
- Selection of study sites and principal investigators
- Trial set-up
- Project management
- Meticulous management of regulatory compliance
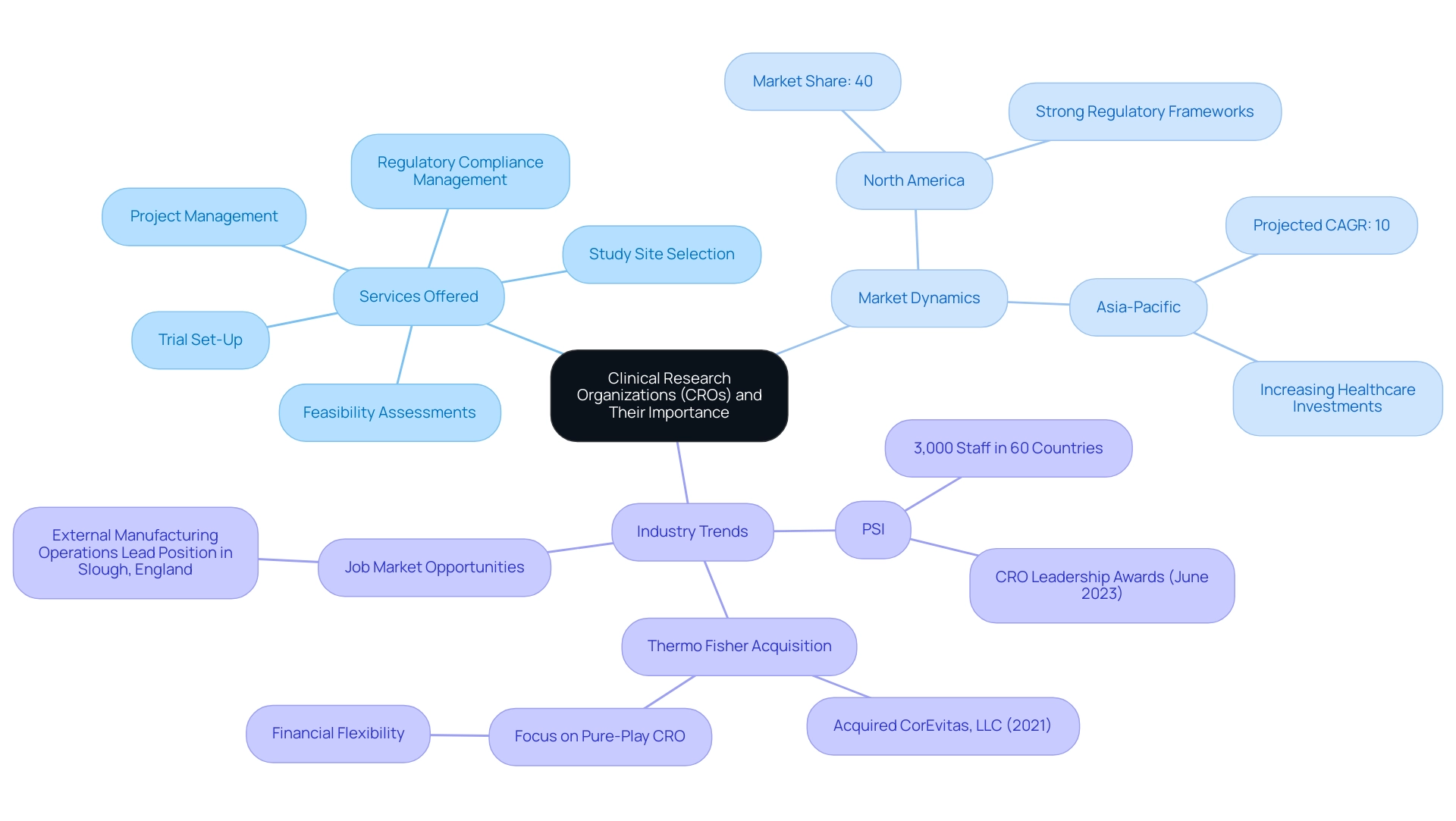
Their expertise guarantees swift regulatory approvals, efficient site activation, and effective subject recruitment, leveraging Colombia's competitive advantages like cost efficiency, speed of regulatory processes, and R&D tax incentives. The significance of the largest clinical research organization is further illustrated by its capacity to streamline processes, reduce costs, and elevate the quality of clinical research.
For instance, North America commanded the largest share of the Healthcare CRO market in 2023, holding approximately 40% of the total market, attributed to a high concentration of pharmaceutical companies and robust regulatory frameworks. Conversely, the Asia-Pacific region is anticipated to witness the most rapid growth, projected to expand at a compound annual growth rate (CAGR) of 10% through 2024, driven by escalating healthcare investments and favorable regulations. Noteworthy developments in the CRO sector include PSI, which has made significant strides as a fast-growing CRO with a workforce of 3,000 across 60 countries.
In June 2023, PSI received CRO Leadership Awards for Expertise, Quality, and Reliability, underscoring its credibility and achievements in the industry. Additionally, the acquisition of CorEvitas, LLC by Thermo Fisher Scientific in 2021 exemplifies the ongoing consolidation and strategic positioning within the industry. As Tom Pike, Chair and CEO of Fortrea, remarked, 'The sale will position Fortrea to focus on growing as a pure-play CRO, with added financial flexibility.'
Furthermore, there is an available External Manufacturing Operations Lead position in Slough, England, highlighting the job market opportunities within the CRO sector. This reflects the broader trend of contract research organizations aiming to enhance their operational focus and financial viability in a competitive market. To discover more about how bioaccess® can enhance your medical device evaluations, BOOK A MEETING today.
A Look at the Largest Clinical Research Organizations
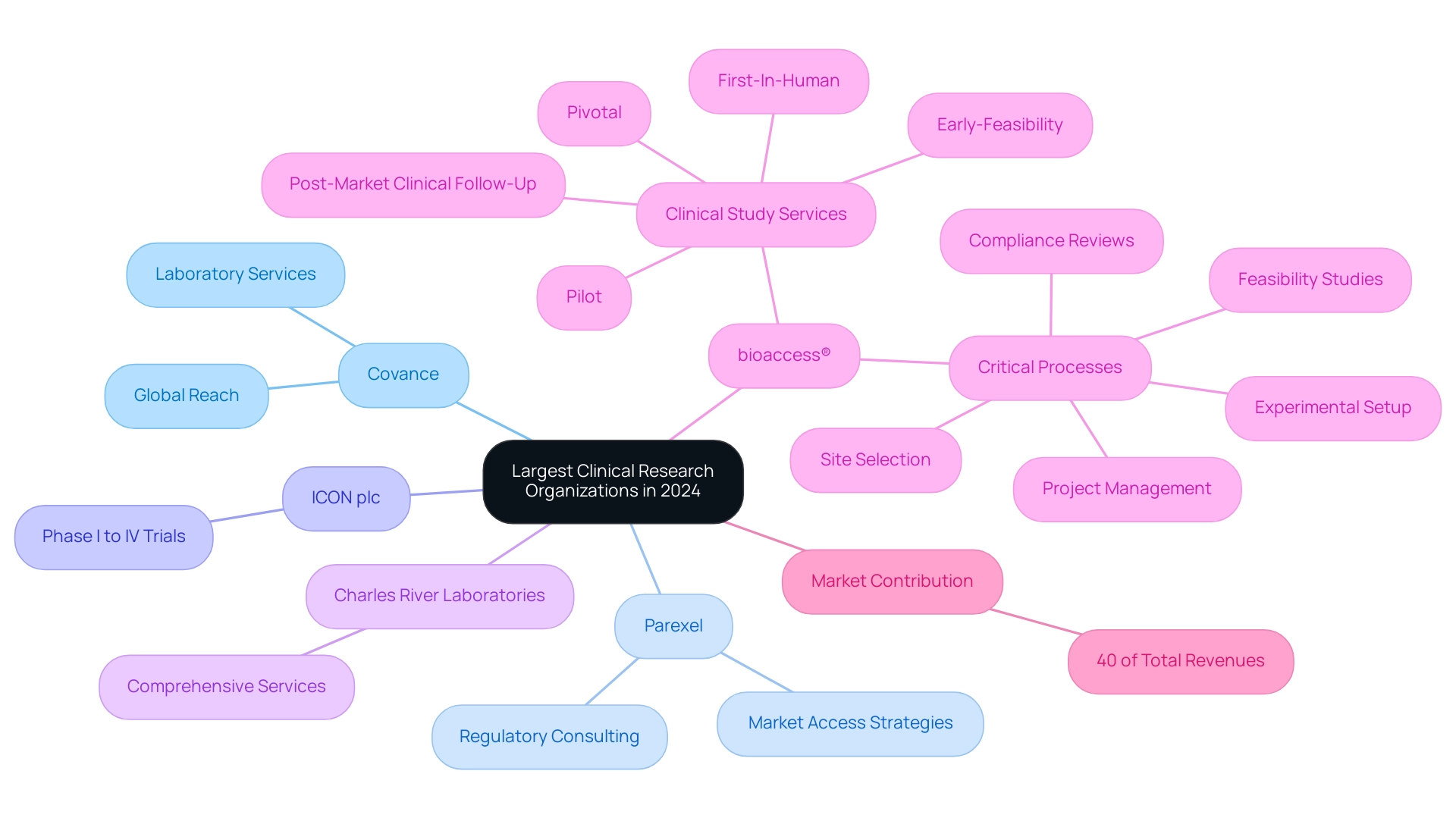
In 2024, Covance, Parexel, ICON plc, and Charles River Laboratories are recognized as some of the largest clinical research organizations (CROs), each providing a diverse array of services that cover all phases from Phase I to Phase IV trials. Covance is particularly noted for its comprehensive laboratory services and extensive global reach, facilitating drug development across multiple regions. In contrast, Parexel excels in regulatory consulting and market access strategies, demonstrating its crucial role in navigating complex regulatory environments.
The medical segment represented a substantial share of the CRO market, accounting for approximately 40% of total revenues in 2024, reflecting the growing demand for specialized services in research. Additionally, bioaccess® is making significant strides in Latin America by providing accelerated medical device clinical study services focused on:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Clinical Follow-Up Studies
Their expertise encompasses critical processes such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Experimental setup
- Project management, including thorough review and feedback on study documents to ensure compliance with country requirements
Furthermore, they prioritize reporting on study status, inventory, and both serious and non-serious adverse events, which are essential for maintaining transparency and regulatory adherence. The collaboration between medical device manufacturers and research institutions fosters innovation and drives global health improvement. Recent initiatives, such as Labcorp's expansion of its automated research kit production line in Belgium and AstraZeneca's launch of Evinova aimed at enhancing access to digital solutions for studies, underscore the ongoing evolution within the field.
As Dr. August Troendle, the CEO, highlights, substantial investments are intended to broaden operations, which will not only generate jobs but also improve the overall effectiveness of medical studies. Together, these advancements emphasize the essential role of the largest clinical research organization, including bioaccess®, in advancing medical research and positively affecting local economies through job creation and healthcare enhancements.
Evolving Roles of CROs in Modern Clinical Trials
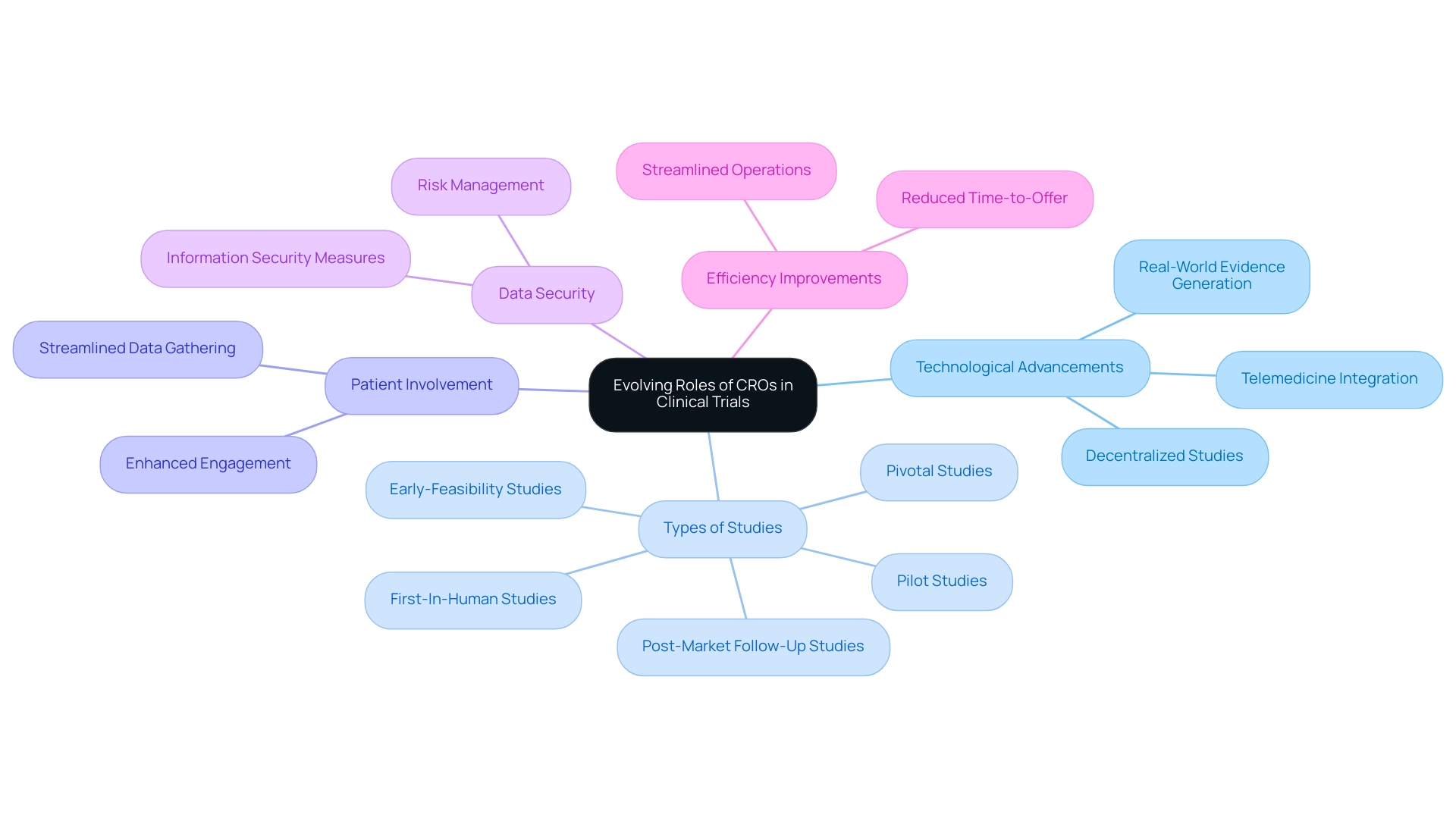
The function of the largest clinical research organization in modern medical studies has experienced a significant change, propelled by technological progress and a movement towards patient-focused approaches. As of June 2024, the count of studies registered in the Western Pacific reached 23,250, roughly 14 times greater than the 845 studies registered in Africa, demonstrating the worldwide expansion of medical research. The largest clinical research organization, CROs, are now essential not only in the management of late-phase studies but also in early-phase research, where they generate real-world evidence and implement adaptive study designs.
A striking trend is the rise of decentralized medical studies, which utilize telemedicine and remote monitoring to facilitate greater patient involvement and streamline data gathering processes. For instance, a recent partnership between a European biotech company and a CRO significantly enhanced study efficiency, reducing the time-to-offer from over 50 days to just 36 days. This emphasizes how innovative methods are transforming the research landscape.
Furthermore, organizations such as bioaccess® are pioneering by offering extensive research management services including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
With more than 20 years of established expertise in Medtech, bioaccess® highlights the specialized knowledge and adaptability necessary to manage the complexities of medical device evaluations, ensuring that they are conducted with the utmost levels of compliance and project management. Moreover, bioaccess® emphasizes the significance of information security within medical device studies, implementing reasonable security measures to protect client data while recognizing inherent risks.
This method not only improves outcomes but also fosters trust with stakeholders, rendering these services essential in contemporary medical studies. As noted by Thermo Fisher Scientific Inc., the integration of Functional Service Provider (FSP) models enhances CRO capabilities. This evolution denotes a wider shift towards adaptable and responsive testing methodologies that emphasize patient experience and involvement, ultimately resulting in more effective outcomes in studies by the largest clinical research organization.
The Role of Technology and AI in Transforming Clinical Research
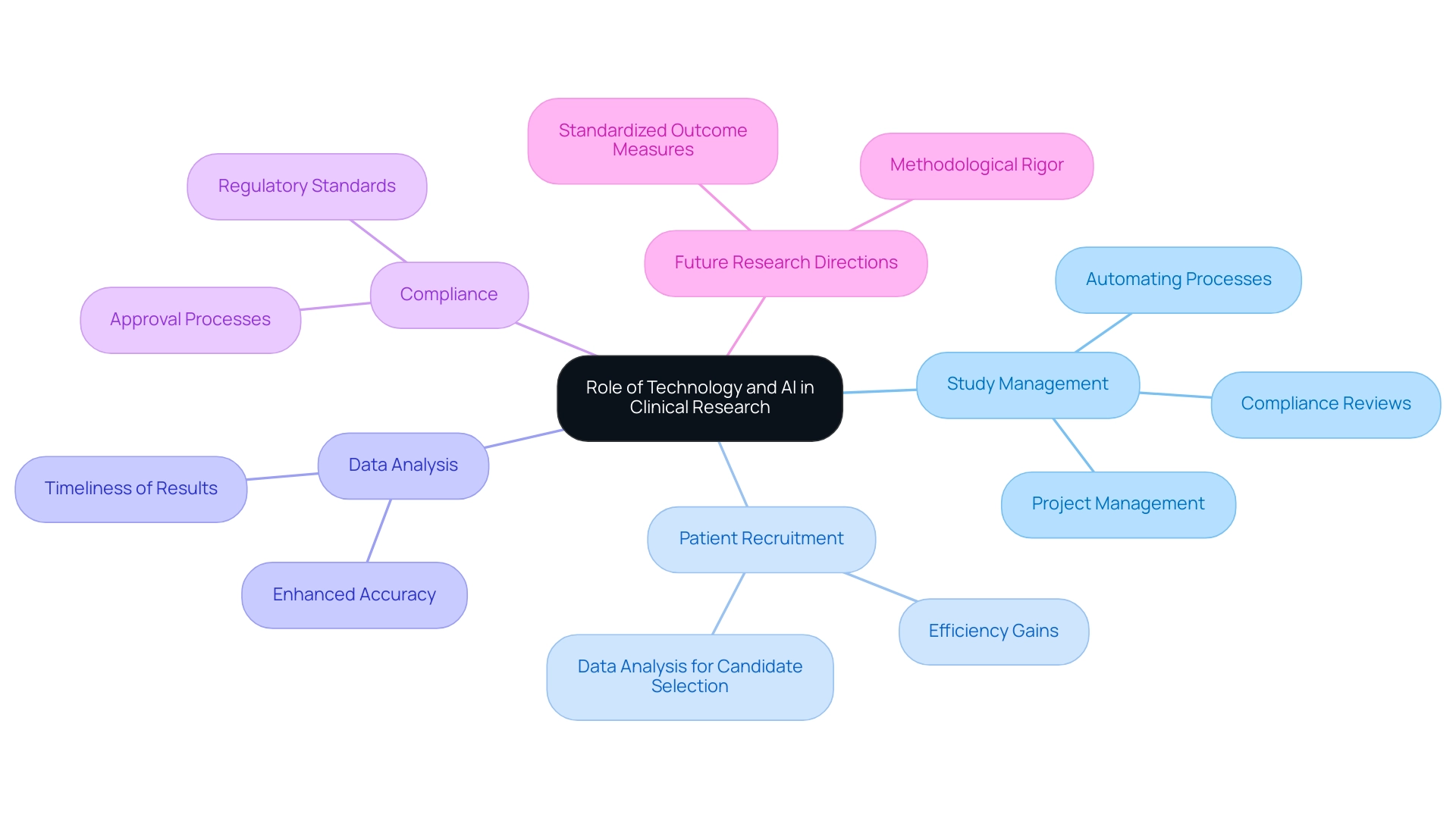
The integration of technology and artificial intelligence (AI) is fundamentally transforming the field of research. Thorough research study management services from the largest clinical research organization—including feasibility studies, site selection, compliance reviews, study setup, and project management—are vital in this transformation. By automating critical processes and enhancing data analysis capabilities, AI is streamlining patient recruitment strategies.
Advanced AI algorithms can efficiently sift through extensive datasets to identify suitable candidates for medical studies, significantly shortening recruitment timelines. Notably, AI can help reach analysis-related milestones in half the time it took without such technology, showcasing its efficiency gains. In 2024, the advent of electronic data capture (EDC) systems has further streamlined data collection and management, resulting in enhanced accuracy and timeliness of results.
Studies indicate that 71% of experiments report marked improvements in outcomes for AI-assisted clinicians compared to their unassisted counterparts. However, as highlighted in the case study titled 'Challenges and Future of AI in Clinical Trials,' the effectiveness of AI depends on the quality of training and user acceptance. Dr. Sergio Alvarado, a Clinical Experiment Manager dedicated to groundbreaking medical studies and AI in Latin America, stresses that future investigations should concentrate on standardized outcome metrics and enhancing methodological precision in studies on AI in medical experiments.
Additionally, the integration of AI is crucial for compliance reviews and the approval processes, ensuring that study documents meet regulatory standards. As Xiaoran Lu aptly stated, the application of AI innovation can foster research transformation and improve the efficacy and quality of medical studies. These advancements not only improve operational efficiency for the largest clinical research organization but also enhance the integrity and reliability of study outcomes, including comprehensive reporting on study status and adverse events, paving the way for a new era in medical investigation.
Challenges and Solutions for Clinical Research Organizations
As the terrain of medical research changes, the largest clinical research organization faces considerable obstacles, particularly in patient recruitment, regulatory compliance, and data management. In 2024, the intricacies of recruitment continue, often worsened by strict eligibility requirements and a general unawareness about the availability and advantages of research studies among potential participants. To combat these hurdles, many CROs are adopting innovative recruitment strategies, such as leveraging social media platforms and patient registries to reach broader audiences and improve visibility.
Moreover, the partnership between bioaccess™ and Caribbean Health Group demonstrates a forward-thinking strategy to improve research studies in Barranquilla, backed by Colombia's Minister of Health, with the goal of establishing the city as a premier location for research in Latin America. This partnership not only emphasizes the significance of international cooperation but also illustrates the potential influence of research studies on local economies, including job creation and healthcare enhancement. bioaccess™ provides a thorough array of services, such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup for experiments
- Import permits
- Project management
- Reporting
These services are vital for maneuvering through the intricacies of medical studies.
The integration of advanced technologies—such as electronic data capture, wearable devices, telemedicine, and artificial intelligence—has become crucial. These tools streamline recruitment processes, minimize manual efforts, reduce data errors, and accelerate study timelines, thus enhancing the overall efficiency of clinical research. A case study titled 'Using Advanced Technology' illustrates how these innovations are applied in practice, demonstrating their impact on trial efficiency.
Compliance with ever-changing regulations remains a top priority, necessitating ongoing training and investment in compliance management systems. Additionally, costs for initiating a medical study in 2024 are not expected to decrease substantially, which adds to the financial pressures faced by the largest clinical research organization. By proactively tackling these complex challenges, clinical research entities can significantly improve their operational efficiency, ultimately resulting in higher-quality study results and increased patient recruitment success rates.
As Debra Anderson, SVP of Strategic Partnerships, remarked, 'Building and managing global teams with innovative strategies is crucial for navigating the challenges in research today.
The Future of CROs: Opportunities in the Medtech Sector
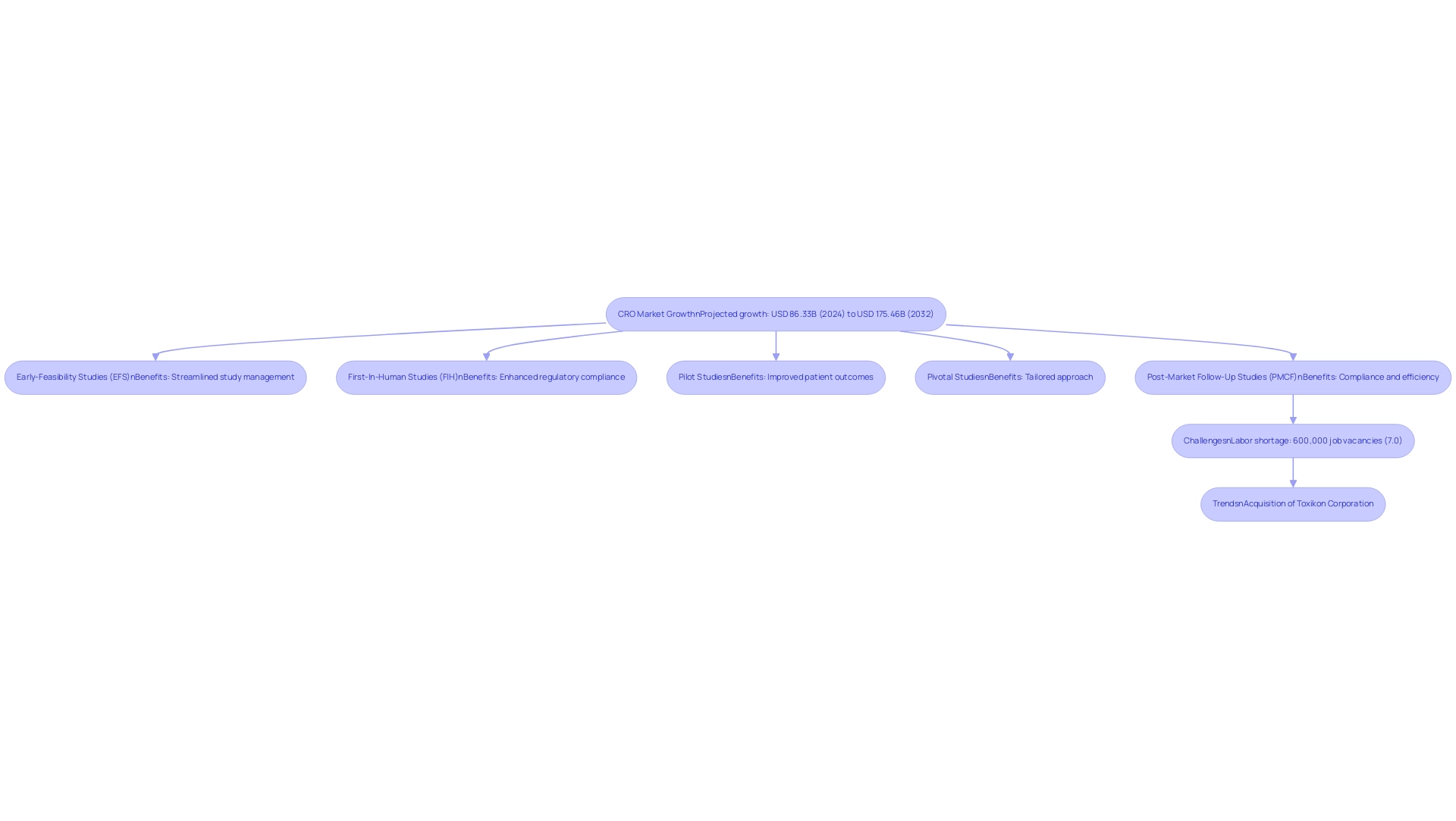
The largest clinical research organization (CRO) landscape is poised for significant growth, particularly within the medical technology (medtech) sector. As the global CRO services market, driven by the largest clinical research organization, is projected to expand from USD 86.33 billion in 2024 to USD 175.46 billion by 2032, the demand for innovative medical devices is set to rise sharply. The largest clinical research organization (CROs) are strategically positioned to offer vital support throughout the development and testing phases of these products.
A prime example is bioaccess®, which focuses on accelerated medical device research services in Latin America. With more than 20 years of experience, bioaccess® excels in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This ensures compliance and efficiency throughout the research process. The benefits of utilizing bioaccess®'s services include streamlined study management, enhanced regulatory compliance, and improved patient outcomes through their tailored approach.
In June 2023, the largest clinical research organization, PSI, was recognized with CRO Leadership Awards for Expertise, Quality, and Reliability, underscoring its commitment to excellence in the field. In areas like Latin America, where funding for healthcare infrastructure is increasing, organizations such as bioaccess® can take on an essential role in supporting research for medtech advancements. Their expertise in navigating regulatory compliance with agencies such as INVIMA not only enhances patient engagement but also accelerates the introduction of new technologies, translating into improved patient care and outcomes.
Moreover, as emphasized by industry specialists, the medtech sector offers numerous opportunities for the largest clinical research organization to contribute significantly to health improvements, reinforcing their importance in the changing environment of research. However, challenges remain, as indicated by Labiotech's report of over 600,000 job vacancies in biopharma, reflecting a labor shortage of 7.0%. This shortage poses significant challenges for the largest clinical research organization in meeting the growing demand for clinical trials.
Additionally, the recent acquisition of Toxikon Corporation by Laboratory Corporation of America Holdings signifies ongoing trends and developments in the CRO industry, further enhancing the relevance of CROs in medical technology.
Conclusion
The role of Clinical Research Organizations (CROs) is increasingly vital in the landscape of clinical trials, serving as the backbone for pharmaceutical and medical device development. By providing specialized services in regulatory compliance, patient recruitment, and data management, CROs facilitate the efficient execution of clinical trials. The growth of the CRO sector, particularly in regions like Latin America, underscores the pressing need for their expertise in navigating the complexities of modern healthcare.
Technological advancements, especially the integration of artificial intelligence and decentralized trial methodologies, are revolutionizing how CROs operate. These innovations not only enhance operational efficiency but also improve patient engagement and data accuracy, ultimately leading to better clinical outcomes. As the industry continues to evolve, the importance of CROs in addressing challenges such as patient recruitment and regulatory compliance cannot be overstated.
Looking ahead, the future of CROs appears promising, particularly within the medical technology sector. With significant market growth projected, CROs are well-positioned to support the development of innovative medical devices, thereby playing a crucial role in advancing healthcare solutions. Their ability to adapt to changing demands and leverage cutting-edge technologies will be essential in meeting the needs of a rapidly evolving clinical research landscape.
In summary, as CROs continue to evolve and expand their capabilities, they will remain indispensable partners in the quest for medical advancements. Their contributions not only enhance the efficiency and quality of clinical trials but also ultimately lead to improved patient outcomes and healthcare innovations. The ongoing collaboration between CROs and pharmaceutical companies is vital for navigating the complexities of clinical research, paving the way for a healthier future.
Frequently Asked Questions
What role do clinical research organizations (CROs) play in medical studies?
CROs offer a diverse range of services to assist in the planning, execution, and management of medical studies, playing a crucial role in the drug development process by providing specialized knowledge in regulatory compliance, patient recruitment, and data management.
How does bioaccess® differentiate itself in the CRO market?
Bioaccess® stands out in Latin America by offering comprehensive services such as feasibility assessments, selection of study sites and principal investigators, trial set-up, project management, and meticulous management of regulatory compliance.
What are the advantages of using CROs like bioaccess® for pharmaceutical firms?
By delegating research studies to CROs, pharmaceutical firms can access specialized expertise, which speeds up the creation of new therapies and improves patient outcomes.
What competitive advantages does Colombia offer for CRO services?
Colombia provides cost efficiency, speed of regulatory processes, and R&D tax incentives, which contribute to swift regulatory approvals, efficient site activation, and effective subject recruitment.
What are some notable trends in the CRO market?
North America holds a significant share of the Healthcare CRO market, while the Asia-Pacific region is expected to grow rapidly due to increasing healthcare investments and favorable regulations.
Who are some of the largest CROs recognized in 2024?
Covance, Parexel, ICON plc, and Charles River Laboratories are among the largest CROs, each offering a range of services across all phases of clinical trials.
What specific services does bioaccess® provide for medical device evaluations?
Bioaccess® offers services including early-feasibility studies, first-in-human trials, pilot studies, pivotal studies, and post-market clinical follow-up studies, along with feasibility studies, site selection, compliance reviews, and project management.
How does bioaccess® ensure compliance and transparency in studies?
They prioritize reporting on study status, inventory, and both serious and non-serious adverse events, which are essential for maintaining transparency and regulatory adherence.
What recent developments highlight the evolution in the CRO sector?
Recent initiatives include Labcorp's expansion of its automated research kit production line in Belgium and AstraZeneca's launch of Evinova to enhance access to digital solutions for studies.
How do CROs contribute to local economies and healthcare advancements?
CROs, including bioaccess®, advance medical research, create jobs, and enhance healthcare, positively impacting local economies.

