Overview
Contract Research Organizations (CROs) in Latin America are specialized entities that provide essential services for managing research studies, including participant recruitment and data management, significantly contributing to the region's medical research landscape. The article highlights Colombia's advantages, such as cost efficiency and a robust healthcare system, which position it as a leading destination for clinical trials, thereby underscoring the growing importance and potential of CROs in advancing research and innovation in Latin America.
Introduction
In recent years, Latin America has emerged as a pivotal player in the realm of clinical research, with Contract Research Organizations (CROs) at the forefront of this transformation. These specialized entities are not only essential for managing the intricate processes of clinical trials but also serve as vital links between pharmaceutical companies and the diverse regulatory landscapes of the region.
Countries like Colombia are setting benchmarks in this field, offering a combination of cost efficiency, rapid regulatory approvals, and high-quality healthcare systems. As the demand for clinical trial management services surges, understanding the dynamics of CROs and the opportunities they present becomes increasingly crucial for stakeholders aiming to navigate the complexities of drug development.
This article delves into the defining features, market trends, core services, and the challenges faced by CROs in Latin America, providing a comprehensive overview of a market poised for significant growth.
Defining Contract Research Organizations (CROs) in Latin America
Contract Research Organizations (CROs) function as specialized entities that provide a broad array of services vital for the planning, execution, and management of research studies. In Latin America, the Contract Research Organization in Latin America plays a crucial role in promoting medical research by utilizing their knowledge in compliance, participant recruitment, and data management. Colombia stands out as a top destination for first-in-human (FIH) studies, boasting competitive advantages such as:
- A 30% cost reduction compared to North America and Western Europe
- Accelerated regulatory review processes (90-120 days)
- A high-quality healthcare system ranked #22 in the world by the World Health Organization
The partnership between bioaccess™ and Caribbean Health Group seeks to elevate Barranquilla's standing as a premier location for research studies, backed by Colombia's Minister of Health, which further highlights the nation's dedication to becoming a center for medical investigations. Furthermore, strategic partnerships with companies like IDx Technologies and Global Care Clinical Trials demonstrate the effectiveness of CROss in improving recruitment times by over 50% and achieving 95% participant retention rates. Notably, investments in science, technology, and innovation projects in Colombia benefit from significant R&D tax incentives, including:
- A 100% tax deduction
- A 25% tax discount
- A 50% future tax credit
This makes it an attractive option for medical device companies.
Furthermore, hospitals in Colombia must undergo a stringent ICH/GCP certification process to carry out research, ensuring high standards of quality assurance. As the market for CRO solutions is anticipated to attain USD 9.47 billion by 2032, investing in a Contract Research Organization in Latin America presents a distinctive opportunity for economical and significant research studies. CROss provide pharmaceutical and biotech companies with the necessary infrastructure and resources to navigate local regulations while upholding international standards, thereby optimizing operational efficiency and enhancing subject enrollment and retention rates.
For further insights, a free sample copy of a relevant report is available upon request.

Market Trends and Growth Factors for CROs in Latin America
The growth of the Contract Research Organization in Latin America market has been significant in recent years, driven by a rising demand for comprehensive management of research studies. These services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting on study statuses and adverse events
However, Medtech companies in the region face significant challenges, including:
- Compliance obstacles
- Language barriers
- Fragmented resources
These challenges complicate their ability to conduct clinical trials effectively.
This growth is largely fueled by enhancements in healthcare infrastructure and the increasing need for efficient drug development processes, prompting pharmaceutical companies to leverage a Contract Research Organization in Latin America for their specialized expertise and local insights. Notably, the CRO market in the APAC MEA region is priced at USD 3800, providing a comparative perspective on market value. A significant trend is the growing emphasis on patient-centric research, which aligns with evolving regulatory standards and patient expectations, facilitating international collaboration.
Furthermore, the adoption of innovative technologies, such as artificial intelligence and machine learning—evident in initiatives like IQVIA's strategic plans—has opened new avenues for the Contract Research Organization in Latin America to refine their service offerings. Such integration is expected to improve the overall quality and efficiency of medical studies, ultimately promoting job creation, economic expansion, and healthcare advancements in the region.
The forthcoming report on the Contract Research Organization in Latin America Services Market, scheduled for release in January 2024 (report code PH 4672), highlights the timeliness and importance of these developments, emphasizing the CRO's role as a crucial contributor to advancing research and innovation.
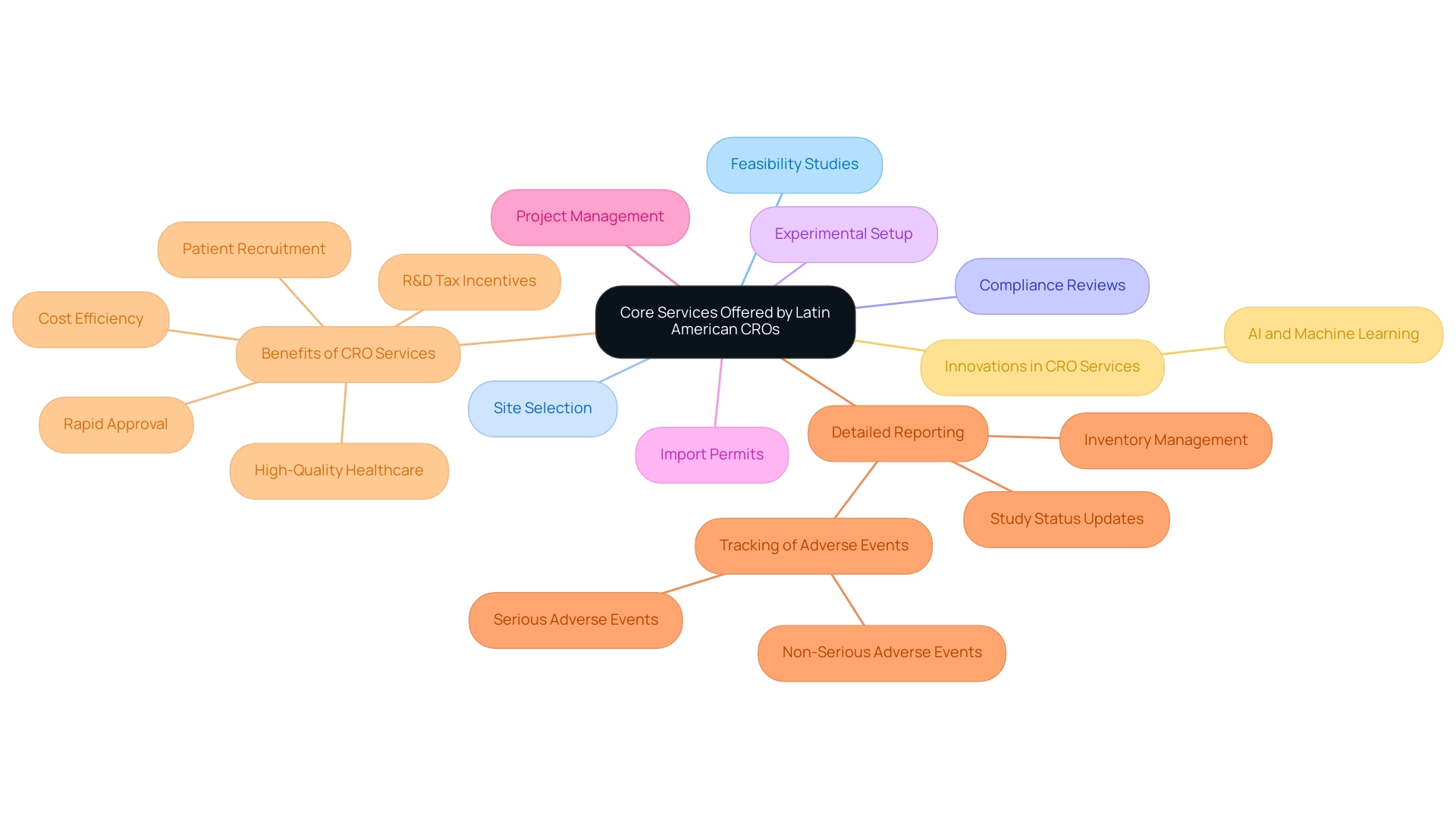
Core Services Offered by Latin American CROs
Contract Research Organizations in Latin America offer a complete range of solutions vital for efficient research management. These services consist of:
- Feasibility studies
- Site selection
- Compliance reviews
- Experimental setup
- Import permits
- Project management
- Detailed reporting, which encompasses:
- Study status updates
- Inventory management
- Tracking of serious and non-serious adverse events
By optimizing the research procedure, CROs guarantee that studies are carried out effectively and comply with strict guidelines established by entities like INVIMA, acknowledged as a Level 4 health authority by PAHO/WHO.
This allows sponsors to focus on their core strengths while upholding a commitment to quality and integrity in execution. For instance, Colombia provides competitive benefits for first-in-human trials, including:
- Cost efficiency
- Rapid approval
- High-quality healthcare
- Patient recruitment
- R&D tax incentives
The integration of these offerings not only enhances operational efficiency but also supports the successful navigation of complex regulatory landscapes, thereby fostering a conducive environment for clinical research by a Contract Research Organization in Latin America.
Recent developments, such as the acquisition of Toxikon Corporation by Laboratory Corporation of America Holdings, further emphasize the increasing importance of strong CRO offerings in advancing medical research. Furthermore, innovations such as IQVIA's strategy to utilize AI and machine learning demonstrate how technological advancements are being employed by CROs to improve quality and efficiency. As emphasized by one leader in the defense industry:
'Thank you for sending the market report and data.
It looks quite comprehensive and the data is exactly what I was looking for. I appreciate the timeliness and responsiveness of you and your team,'
underscoring the importance of comprehensive data and responsiveness in CRO services.
Challenges and Opportunities for CROs in the Region
Contract Research Organizations in Latin America face a myriad of challenges that can hinder their effectiveness, including complex regulatory environments and infrastructure limitations. For example, while statistics show that Haiti has finished 0.0% of research studies, emphasizing considerable operational difficulties, Colombia emerges as a symbol of advancement in the area. Our service capabilities encompass:
- Feasibility and selection of research sites and principal investigators (PIs)
- Compliance reviews of study documents
- Trial setup, including approvals from ethics committees and health ministries
Moreover, we facilitate import permits and the nationalization of investigational devices, ensuring that all processes align with local regulations overseen by INVIMA, Colombia's National Food and Drug Surveillance Institute, classified as a Level 4 health authority by PAHO/WHO. These capabilities not only support compliance but also contribute to local economies through:
- Job creation
- Economic growth
- Healthcare improvement
Our reporting processes are designed to keep stakeholders informed, detailing study status and managing both serious and non-serious adverse events effectively.
As Julio G. Martinez-Clark, co-founder and CEO of bioaccess, aptly states, 'Thanks to the efforts made by the different actors in the health sector, Colombia is a powerhouse and a prominent Contract Research Organization in Latin America for medical research.' By investing in local partnerships, which are crucial for enhancing the effectiveness of CROss, and leveraging advanced technologies, we can effectively navigate these compliance challenges and foster international collaboration, ensuring sustainable growth in 2024 and beyond.
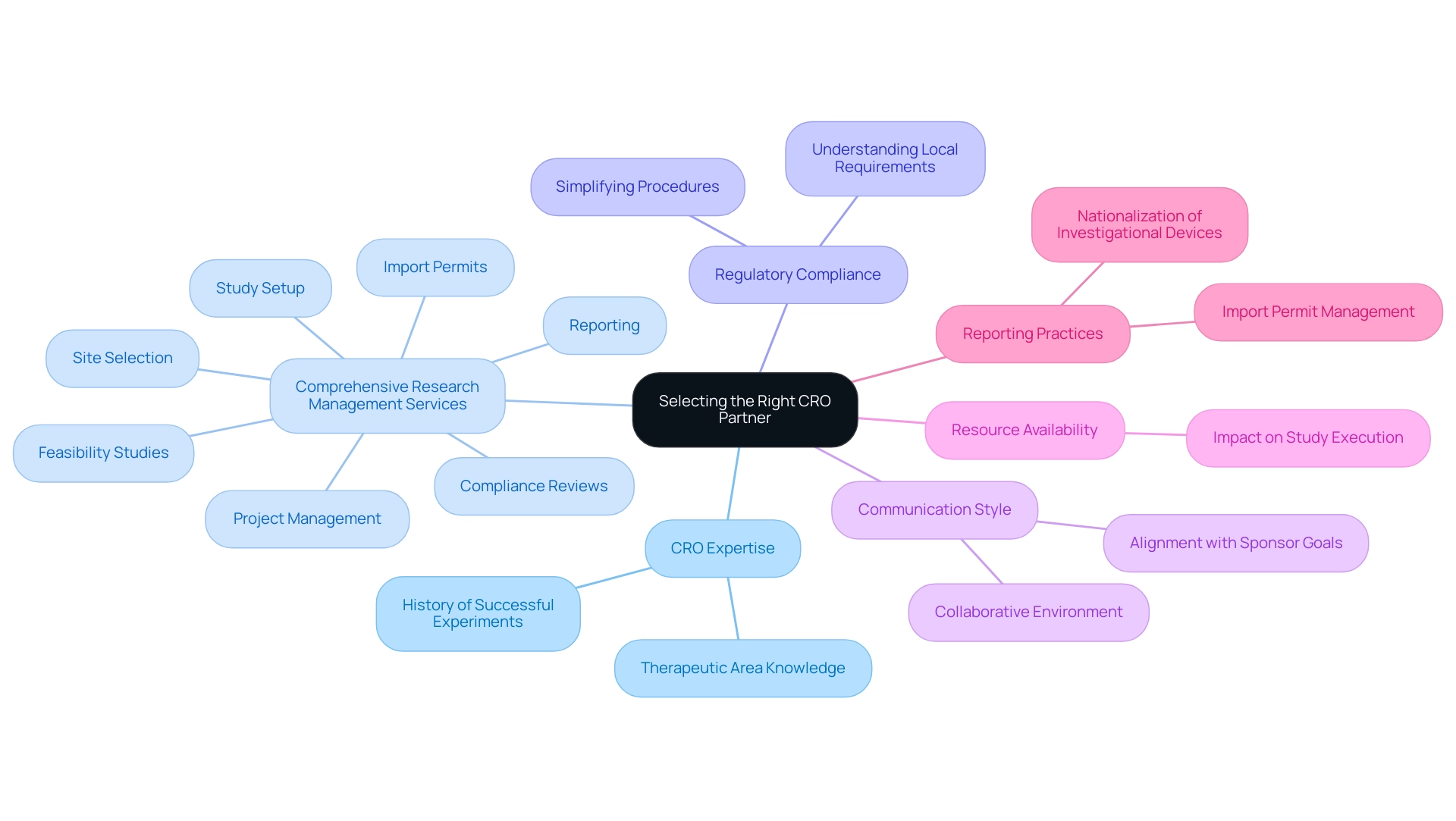
Selecting the Right CRO Partner: Key Considerations
Selecting the appropriate Contract Research Organization in Latin America (CRO) partner is pivotal for the success of clinical research projects. Stakeholders should meticulously evaluate several key factors, including the CRO’s expertise in the specific therapeutic area, which is essential for navigating complex study requirements and regulatory landscapes. Their established history in overseeing successful experiments serves as a sign of reliability and effectiveness.
Comprehensive research management services, such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
are essential elements to consider. A thorough comprehension of local regulatory requirements improves adherence and simplifies procedures, especially in areas like Australia, where numerous biotechnology firms perform early-stage studies because of advantageous regulatory conditions. Evaluating the CRO's communication style and project management capabilities is also vital; these elements foster a collaborative environment that aligns with the sponsor's goals.
Resource availability should not be overlooked, as it directly impacts the ability to execute studies efficiently. Forming a strong alliance with a suitable Contract Research Organization in Latin America can greatly enhance the efficiency and success of research initiatives, ultimately leading to improved patient outcomes. Given that 88% of post-Phase I studies engage CROs, especially among smaller pharmaceutical companies, the right partnership can be a game-changer in navigating the complexities of clinical trials.
Additionally, attention to reporting practices and the import permit and nationalization of investigational devices is critical for ensuring smooth operations throughout the study.
Conclusion
The evolution of Contract Research Organizations (CROs) in Latin America marks a significant development in the field of clinical research, establishing the region as a key player in global drug development. The article highlights the essential role of CROs in facilitating clinical trials through their comprehensive services, which include:
- Feasibility studies
- Site selection
- Compliance management
Particularly, Colombia emerges as a standout location, offering cost-effective solutions, rapid regulatory approvals, and a commitment to high-quality healthcare, making it an attractive destination for pharmaceutical companies seeking to conduct trials.
As the CRO market continues to expand, driven by the increasing demand for efficient clinical trial management, stakeholders must recognize both the opportunities and challenges present in the region. The integration of advanced technologies, such as artificial intelligence, is reshaping the landscape, enhancing the quality and efficiency of clinical trials. However, navigating regulatory complexities and infrastructure limitations remains a critical concern.
Ultimately, the success of clinical research initiatives hinges on the careful selection of CRO partners. By considering factors such as:
- Expertise
- Track record
- Local regulatory knowledge
Stakeholders can forge effective collaborations that not only streamline the trial process but also contribute to the overall growth of the healthcare sector in Latin America. The potential for significant advancements in clinical research is evident, positioning the region as a vital contributor to global health innovation.
Frequently Asked Questions
What are Contract Research Organizations (CROs)?
CROs are specialized entities that provide a wide range of services essential for the planning, execution, and management of research studies.
What role do CROs play in Latin America?
In Latin America, CROs promote medical research by utilizing their expertise in compliance, participant recruitment, and data management.
Why is Colombia considered a top destination for first-in-human (FIH) studies?
Colombia is favored for FIH studies due to a 30% cost reduction compared to North America and Western Europe, accelerated regulatory review processes (90-120 days), and a high-quality healthcare system ranked #22 globally by the World Health Organization.
What partnerships are enhancing research opportunities in Colombia?
The partnership between bioaccess™ and Caribbean Health Group aims to elevate Barranquilla's status for research studies, supported by Colombia's Minister of Health. Collaborations with companies like IDx Technologies and Global Care Clinical Trials have improved recruitment times by over 50% and achieved 95% participant retention rates.
What tax incentives are available for investments in science and technology in Colombia?
Investments benefit from significant R&D tax incentives, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit.
What standards must hospitals in Colombia meet to conduct research?
Hospitals must undergo a stringent ICH/GCP certification process, ensuring high standards of quality assurance in research.
What is the projected market value for CRO solutions by 2032?
The market for CRO solutions is anticipated to reach USD 9.47 billion by 2032.
What services do CROs provide for pharmaceutical and biotech companies?
CROs offer essential infrastructure and resources to navigate local regulations while maintaining international standards, optimizing operational efficiency, and enhancing subject enrollment and retention rates.
What challenges do Medtech companies face in Latin America?
Medtech companies encounter compliance obstacles, language barriers, and fragmented resources, complicating their ability to conduct clinical trials effectively.
What trends are influencing the CRO market in Latin America?
The market is influenced by enhancements in healthcare infrastructure, a focus on patient-centric research, and the adoption of innovative technologies like artificial intelligence and machine learning.
When is the forthcoming report on the Contract Research Organization in Latin America Services Market scheduled for release?
The report is scheduled for release in January 2024.




