Introduction
The emergence of De Novo devices marks a significant evolution in the medical device landscape, offering a pathway for innovative technologies that address unmet clinical needs. Recognized by the U.S. Food and Drug Administration (FDA) as low to moderate risk, these devices pave the way for novel solutions that enhance patient care and improve health outcomes.
As healthcare continues to advance, the De Novo classification process plays a crucial role in facilitating the introduction of groundbreaking medical technologies. This article delves into the intricacies of the De Novo pathway, exploring its importance, the challenges faced by manufacturers, and the future trends that are set to shape the development of these transformative devices.
Through a comprehensive examination of recent advancements and regulatory updates, the discussion underscores the pivotal role of De Novo devices in modern healthcare and their potential to drive innovation on a global scale.
Defining De Novo Devices: An Overview
These products represent an essential category of healthcare tools acknowledged by the U.S. Food and Drug Administration (FDA) as low to moderate risk, characterized by their innovative nature. This classification is essential as it facilitates the marketing of products lacking a substantially equivalent counterpart currently available on the market. The de novo device pathway is specifically designed for groundbreaking products with the potential to greatly improve patient care by meeting unfulfilled health requirements.
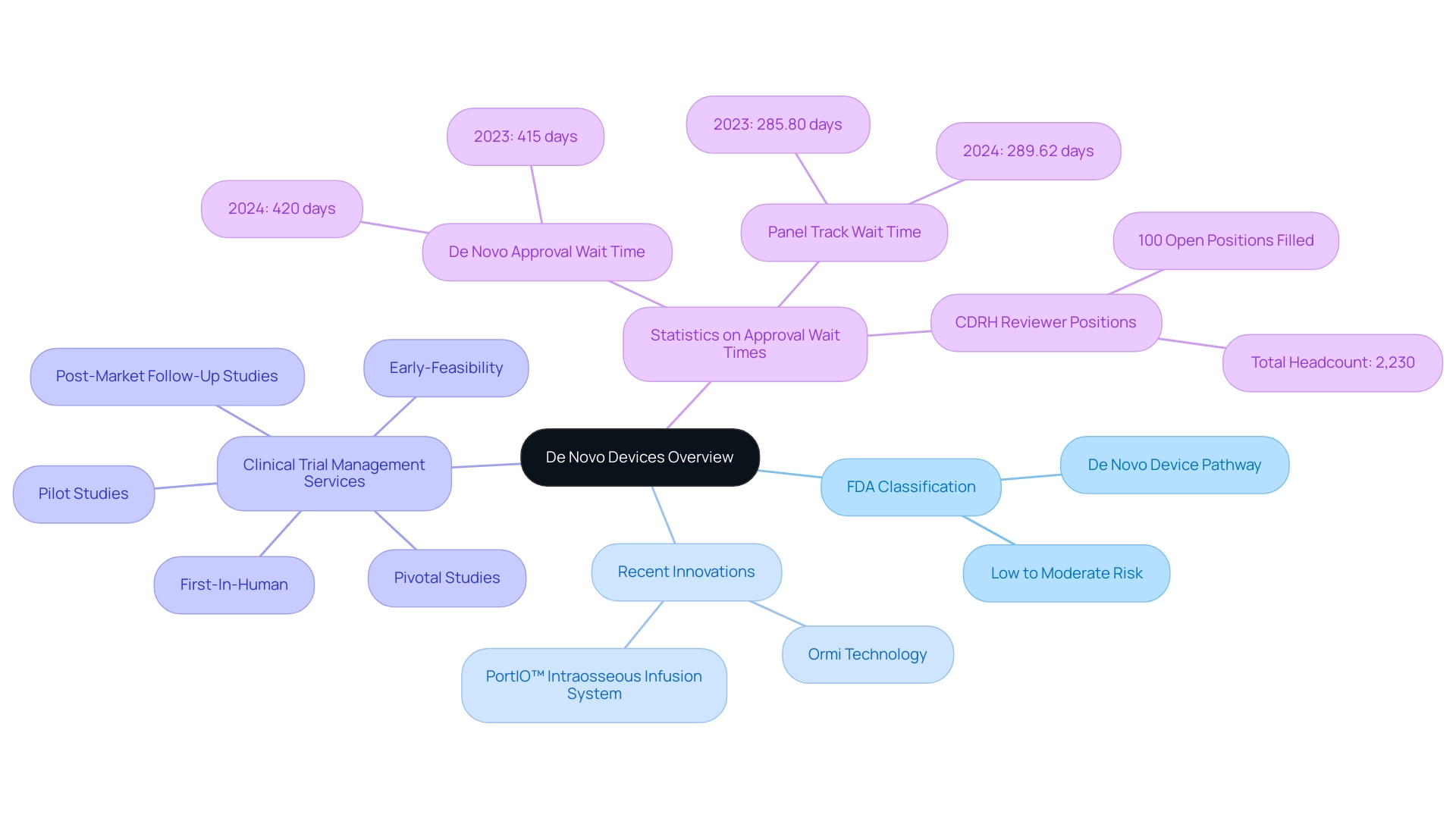
Recent advancements, such as the treatment of initial patients with the breakthrough Ormi technology by Sparta Biomedical, exemplify how these innovations can reshape healthcare outcomes. Additionally, PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia have demonstrated successful outcomes, showcasing the importance of rigorous clinical trial management. By simplifying the approval procedure for these innovative technologies, the FDA not only encourages the advancement of new healthcare solutions but also ultimately improves health outcomes for patients and providers alike.
Furthermore, comprehensive clinical trial management services, like those offered by bioaccess®, ensure effective oversight of:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
These services include feasibility studies, site selection, compliance reviews, and project management, reinforcing their significance in the success of clinical trials. In 2024, the FDA's commitment to supporting medical innovation is evident in its statistics, which underscore an ongoing dedication to approving new devices that contribute meaningfully to patient health.
However, it is noteworthy that new authorization wait times have increased slightly from 415 days in 2023 to 420 days in 2024, indicating variability in the regulatory process. Additionally, Panel Track wait times rose from 285.80 days in 2023 to 289.62 days in 2024. The CDRH has also achieved a significant milestone by filling 100% of its open reviewer positions for the 2023 fiscal year, achieving a total headcount of 2,230, which underscores the FDA's operational capacity to manage new applications effectively.
This pathway is increasingly acknowledged as crucial in the realm of healthcare innovation, especially for the regulatory approval of a de novo device.
Navigating the De Novo Classification Process
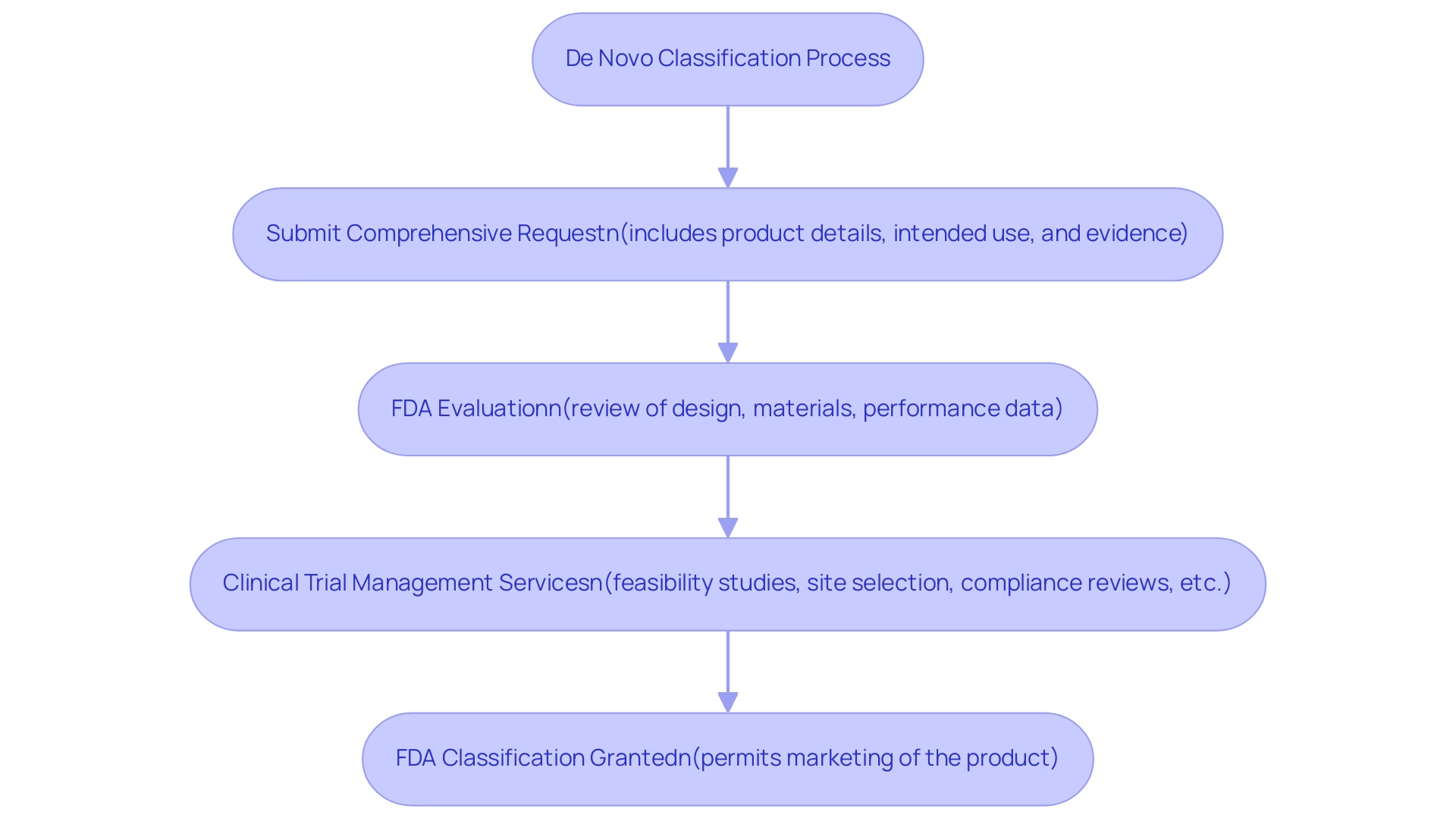
The new classification process includes several crucial steps that manufacturers must follow to obtain FDA approval for their medical instruments. Initially, a manufacturer is required to submit a comprehensive request for a de novo device, which must include detailed information regarding the product, its intended use, and substantial evidence supporting its safety and effectiveness. This submission is essential, as it provides the FDA important data to evaluate the product against established safety and efficacy criteria.
Factors such as the item's design, materials, and performance data are meticulously reviewed during this evaluation phase. Significantly, all content supporting the new request must be in or translated into English, ensuring clear communication of the product's details.
To improve the chances of successful submissions, participating in thorough clinical trial management services is crucial. This encompasses:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Nationalization of investigational equipment
- Project management
These elements are all crucial in navigating the regulatory landscape. Experts such as Katherine Ruiz, specializing in Regulatory Affairs for health products and in vitro diagnostics in Colombia, play a crucial role in guiding manufacturers through these processes.
Once the FDA determines that the product meets safety and effectiveness standards, it grants classification as a de novo device, which permits the manufacturer to market the product. This categorization method not only accelerates the launch of groundbreaking health devices into the market but also guarantees compliance with strict safety standards, thereby protecting public health. Furthermore, recent discussions emphasize the development of novel statistical methods to enhance the identification of risk genes linked to birth defects.
For example, Andrews et al. found that ASD-associated SNPs in GWAS are enriched for tissue-specific meQTLs in fetal brain and peripheral blood, indicating an ongoing commitment to improving the regulatory landscape for medical devices.
In a notable case, the FDA recognized the importance of technical consistency in submissions, leading to a revision in requirements to include protocols and reports for all studies related to nonclinical evaluations. This adjustment highlights the FDA's responsiveness to feedback and its commitment to upholding high standards in the review.
The scale of data involved in the classification procedure is illustrated by the EncoreDNM study, which utilized 31,058 DD trios, 6,430 ASD trios, and other significant data sets. Additionally, the reporting aspect is critical; manufacturers must monitor and report study status, including serious and non-serious adverse events, to ensure ongoing compliance and safety. As manufacturers navigate this complex process, understanding these steps is vital for successful new submissions.
The Importance of De Novo Devices in Modern Healthcare
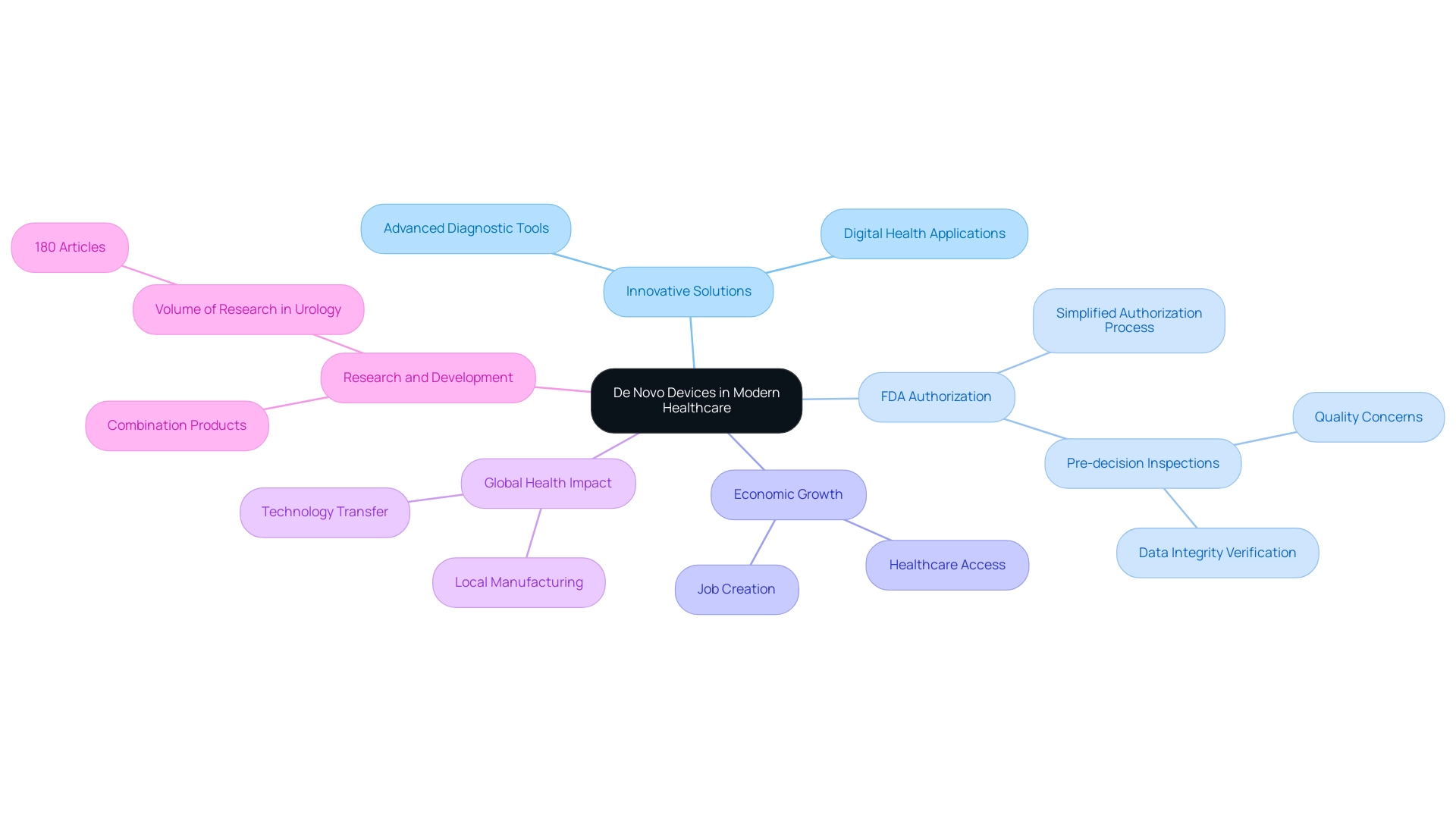
New technologies are crucial in transforming contemporary healthcare by enabling the creation and implementation of inventive solutions that significantly improve patient care. Notably, technologies such as digital health applications and advanced diagnostic tools have emerged as de novo devices through the De Novo pathway, specifically targeting health challenges that traditional systems may overlook. These innovations not only expand treatment options but also promote a tailored approach to medicine, allowing for personalized strategies in patient management.
The FDA's simplified authorization method for de novo devices fosters a climate that promotes creativity, which is vital for enhancing health results. Furthermore, as our Medtech associates conduct clinical studies in various countries, they foster job creation and economic growth while improving healthcare access and outcomes. This procedure also encourages enhanced research and development related to de novo devices, resulting in global acknowledgment of progress in healthcare technology.
The World Health Organization highlights the importance of local manufacturing and technology transfer to enhance access to medical equipment in low- and middle-income nations, emphasizing the worldwide effect of new products. In the field of urology, for instance, there are currently 180 articles, underscoring the volume of research and interest in this area. As emphasized by James L. Johnston from Yale School of Medicine, the FDA's historical method, which seldom required postmarket studies for these items, has established the foundation for new models and rival products that later obtain approval via the 510(k) pathway.
Furthermore, the final rule affirms that the new approach can be applied to combination products, taking safety and effectiveness into account as a whole, while permitting pre-decision inspections. This highlights the crucial role new technologies play in healthcare innovation and their wider effect on local economies and global health enhancement efforts as we progress into 2024 and beyond. The interconnectedness of these advancements is symbolized by our visual representation of a globe, reflecting our commitment to international collaboration in driving healthcare improvements.
Challenges in the De Novo Classification Process
The de novo device classification method offers a considerable opportunity for producers of cutting-edge medical equipment; however, it is not without its difficulties. A primary obstacle lies in the necessity for comprehensive clinical data to substantiate both the safety and effectiveness of instruments. This requirement can be particularly daunting for smaller companies with limited resources, as collecting and analyzing clinical data is both time-consuming and costly.
Notably, a study published in JAMA Internal Medicine analyzed 63 FDA De Novo device authorizations and found that:
- One-fifth of the products were not evaluated in pivotal studies.
- One-third failed to meet at least one primary effectiveness endpoint.
These findings highlight the critical importance of robust clinical data in the approval process. Furthermore, the evolving regulatory landscape, including European reforms aimed at harmonizing evidence standards, adds layers of complexity and uncertainty regarding compliance, compelling manufacturers to maintain continuous dialogue with the FDA.
To successfully navigate these challenges, particularly in Latin America, engaging with experts such as those from bioaccess® can be invaluable. Their comprehensive clinical trial management services include:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
These services equip manufacturers to overcome hurdles effectively. bioaccess®'s customized approach ensures that each trial is tailored to meet specific needs, enhancing the likelihood of success.
Staying informed of the latest guidelines and best practices, including the necessity for postmarket studies to be made publicly available, is crucial for ensuring successful approval and advancing medical trials.
Future Trends in De Novo Device Development
The progress of technology is poised to change the environment for new products significantly, especially in Latin America where expedited clinical study services by bioaccess® are crucial. With expertise in managing:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
bioaccess® is well-positioned to navigate the complexities of clinical trials, ensuring compliance and successful outcomes. Our service capabilities include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Notable trends, including the integration of artificial intelligence, remote monitoring capabilities, and telehealth applications, are expected to influence the types of products that will be classified as a de novo device. As highlighted in the 18th annual Pulse of the MedTech Industry report, the MedTech sector is actively pursuing innovations aimed at creating devices that are not only smarter and smaller but also more personalized to meet patient needs. The report finds that the industry is pushing at new frontiers, with ongoing breakthroughs in MedTech AI and renewed hope for MedTech M&A.
Regulatory bodies, including INVIMA, which is classified as a Level 4 health authority by PAHO/WHO, are acknowledging these advancements, leading to the potential for streamlined processes and more adaptable guidelines. Additionally, insights from the case study titled 'Accelerating New Product Development in 2024' reveal that:
- 86% of industry professionals are optimistic about revenue growth
- 35% prioritizing market approval
- 45% launching new clinical activities this year
Dr. Sergio Alvarado, our Clinical Trial Manager, concentrates on innovative health research and artificial intelligence in Latin America, further enhancing our capabilities.
With 63% of medtech leaders anticipating that digital therapeutics will significantly impact the industry over the next decade, the introduction of de novo devices is poised to be instrumental in facilitating the swift release of pioneering medical devices. This pathway is crucial for addressing the increasing demand for personalized and accessible healthcare solutions, further aligning with the goals of both patients and healthcare providers.
Conclusion
The exploration of De Novo devices reveals their transformative role in the medical device landscape, particularly in enhancing patient care and addressing unmet clinical needs. By offering a streamlined pathway for innovative technologies, the De Novo classification process not only accelerates market access for low to moderate risk devices but also emphasizes the importance of rigorous clinical trial management to ensure safety and efficacy. Recent advancements, as demonstrated by successful implementations of novel devices, underscore the potential of these technologies to reshape healthcare outcomes globally.
However, the journey through the De Novo classification process is not without challenges. Manufacturers face the daunting task of compiling comprehensive clinical data, which can be especially burdensome for smaller companies. The evolving regulatory landscape necessitates ongoing communication with the FDA and a keen understanding of compliance requirements. By leveraging specialized clinical trial management services, manufacturers can navigate these complexities more effectively, enhancing their chances of successful device approval.
Looking ahead, the future of De Novo devices is bright, with technological advancements poised to further revolutionize healthcare delivery. The integration of artificial intelligence, telehealth applications, and personalized medical solutions will likely dominate the landscape, reinforcing the significance of the De Novo pathway in bringing cutting-edge innovations to market. As the MedTech industry continues to evolve, the collaborative efforts of regulatory bodies and manufacturers will be crucial in driving the development of devices that meet the increasing demands for accessible and tailored healthcare solutions. Through these advancements, the De Novo classification process stands as a vital mechanism for fostering innovation and improving health outcomes on a global scale.
Frequently Asked Questions
What is the de novo device pathway?
The de novo device pathway is a regulatory process designed by the FDA for innovative medical products that are classified as low to moderate risk. It allows manufacturers to market products that do not have a substantially equivalent counterpart currently available on the market, facilitating the introduction of groundbreaking healthcare solutions.
What are the key benefits of the de novo device pathway?
This pathway encourages the advancement of new healthcare technologies by simplifying the approval process, ultimately improving health outcomes for patients and providers. It is particularly significant for products that meet unfulfilled health requirements.
What recent advancements illustrate the impact of the de novo device pathway?
Recent advancements include the treatment of patients using Ormi technology by Sparta Biomedical and the first-in-human implantations of the PortIO™ Intraosseous Infusion System by PAVmed in Colombia, both demonstrating successful outcomes and the importance of clinical trial management.
What services do clinical trial management companies like bioaccess® provide?
Clinical trial management services include oversight of early-feasibility studies, first-in-human trials, pilot studies, pivotal studies, and post-market follow-up studies. They also offer feasibility studies, site selection, compliance reviews, and project management to ensure the success of clinical trials.
How has the FDA's approval wait time changed recently?
The wait time for new authorizations has slightly increased from 415 days in 2023 to 420 days in 2024. Similarly, Panel Track wait times rose from 285.80 days in 2023 to 289.62 days in 2024.
What is required for a manufacturer to obtain FDA approval for a de novo device?
Manufacturers must submit a comprehensive request that includes detailed information about the product, its intended use, and evidence supporting its safety and effectiveness. All supporting content must be in or translated into English.
What role do experts play in the de novo device approval process?
Experts in regulatory affairs, such as Katherine Ruiz, guide manufacturers through the submission process, helping with feasibility studies, site selection, compliance reviews, and overall project management to navigate the regulatory landscape effectively.
What happens after the FDA approves a de novo device?
Once the FDA determines that the product meets safety and effectiveness standards, it grants classification as a de novo device, allowing the manufacturer to market the product while ensuring compliance with safety standards to protect public health.
What recent changes have been made to the submission requirements for FDA classification?
The FDA has revised its requirements to include protocols and reports for all studies related to nonclinical evaluations, emphasizing the importance of technical consistency in submissions.
Why is ongoing monitoring and reporting critical for manufacturers?
Manufacturers must monitor and report the status of their studies, including any serious and non-serious adverse events, to ensure ongoing compliance and safety of their medical devices in the market.

