Introduction
In the realm of clinical research, the selection of primary endpoints stands as a cornerstone in the evaluation of treatment efficacy and safety. These endpoints not only guide the design and execution of clinical trials but also play a pivotal role in regulatory decision-making and patient care outcomes.
As the landscape of medical research evolves, understanding the nuances of different types of primary endpoints—ranging from clinical to surrogate measures—has become increasingly critical. The implications of endpoint selection extend beyond statistical analysis; they influence the credibility of trial results and the advancement of medical knowledge.
With the growing complexity of clinical trials and the introduction of innovative methodologies, such as dual and composite endpoints, the need for a comprehensive approach to endpoint definition and measurement is more pressing than ever.
This article delves into the intricacies of primary endpoints, exploring their significance, challenges, and the evolving concepts that shape contemporary clinical research practices.
Defining Primary Endpoints: A Fundamental Overview
Main objectives are the crucial results that a research study is strategically crafted to assess, serving as the fundamental basis for evaluating the effectiveness of therapies or interventions. The meticulous choice of a primary endpoint meaning is crucial, as it directly influences the study's design, statistical evaluation, and the subsequent interpretation of findings. Instances of main outcomes frequently observed in research studies include:
- Overall survival rates
- Disease progression-free survival
- Relief of symptoms in patients
The U.S. FDA highlights the importance of these objectives, stating in its 2023 guidance on decentralized studies, 'the selection of objectives must be directed by scientific evidence and relevance to patient care.' Understanding the primary endpoint meaning is essential not only for evaluating the effectiveness of clinical studies but also for their wider contributions to enhancing medical knowledge. As sponsors assess whether increases in experimental complexity are necessary, the significance of carefully defining primary objectives becomes even more pronounced.
Moreover, with 22,825 drugs in the R&D pipeline as of 2024, this careful consideration can significantly influence study outcomes and regulatory decisions. Furthermore, examining trial participants' demographics by gender and ethnicity can offer insights into outcome selection and its implications.
Types of Primary Endpoints in Clinical Research
Main objectives in medical research are crucial for evaluating the effectiveness of therapies and can be classified into various unique categories:
- Clinical Targets: These measures are directly associated with patient results, encompassing metrics such as survival rates and health-related quality of life. They provide clear, observable evidence of a treatment’s effectiveness.
- Surrogate Measures: Acting as indirect indicators, surrogate measures are expected to predict clinical benefits but do not directly reflect patient outcomes. Common examples include laboratory results, such as cholesterol levels, or imaging findings that suggest treatment efficacy. As Dr. John Lawrence from the FDA's Office of Biostatistics cautions, analyzing multiple outcomes within a single trial can provide valuable information but raises concerns regarding 'multiplicity' and the potential for false conclusions. This emphasizes the necessity for careful selection of substitute measures to ensure accuracy in findings.
- Composite Measures: These include several individual measures, enabling researchers to capture a wider range of treatment effects. By merging different results, composite measures can improve the effectiveness of a medical study, especially when singular occurrences are uncommon.
- Time-to-Event Endpoints: This category focuses on measuring the duration until a specific event, such as disease recurrence or patient death, occurs. These measures are especially beneficial in chronic illness research where the timing of results is crucial.
Each category of primary measure is deliberately selected according to the particular goals of the clinical investigation and reflects the primary endpoint meaning of the disease under examination. Current trends suggest that the use of substitute measures is becoming more prevalent, especially with the growth of digital health technologies and connected devices in studies, such as continuous glucose monitors (CGMs) and wearable electrocardiogram (ECG) patches. Furthermore, an examination of study complexity by treatment area indicates that oncology studies have traditionally been the most intricate, which affects outcome selection and study design.
Comprehending these intricacies is vital for maintaining the integrity of research outcomes.
The Importance of Endpoint Selection in Clinical Trials
The choice of an appropriate primary endpoint meaning is crucial for the success of any research study. It is essential that the selected target corresponds with the study's specific goals and produces valuable information that can impact clinical decision-making. A clearly specified objective, reflecting the primary endpoint meaning, not only improves the statistical strength of the study but also plays a crucial role in aiding regulatory approval, ultimately enhancing patient care.
Moreover, the clarity and relevance of the outcome can significantly bolster stakeholder confidence, encompassing sponsors, regulatory bodies, and the broader medical community. On the other hand, inadequately specified targets may result in unclear outcomes, thus compromising the study's reliability and its possible contributions to enhancing medical knowledge, particularly regarding the primary endpoint meaning. Recent conversations among regulatory specialists highlight the importance of accurate target selection, stressing its significance in ongoing research and developing medical practices.
Our extensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Reporting processes
These services ensure that goals are aligned with study aims to enhance patient results. For example, in studies involving hematopoietic stem cell transplantation (HCT), significant results such as issues like acute and chronic graft-versus-host disease are assessed, emphasizing the significance of precise selection of objectives. As the scenery of research studies evolves, comprehending the effect of goal clarity on study results remains a crucial priority for stakeholders in the domain, aiding not only in scientific progress but also in local economies via job creation and enhancement of healthcare.
Challenges in Defining and Measuring Primary Endpoints
Establishing and assessing key targets in clinical trials can present difficulties that greatly influence the primary endpoint meaning of the study results. A predominant issue lies in ensuring that these points reflect the primary endpoint meaning, being both clinically relevant and quantifiable. Subjective measures, such as patient-reported outcomes, exemplify this complexity, as they can be heavily influenced by individual perceptions and may vary widely across participants.
The timing of measurement at the conclusion also plays a critical role; for instance, assessing disease progression at different intervals may lead to inconsistent data that affects the primary endpoint meaning, complicating the interpretation of results. To navigate these challenges, comprehensive research study management services are essential. These consist of:
- Feasibility studies
- Site selection
- Compliance reviews
- Experiment setup
- Import permits
All of which ensure that selected targets adhere to regulatory requirements and industry standards.
Moreover, the implementation of flexible study designs permits adjustments based on gathered information, increasing outcome sensitivity and possibly enhancing clinical development effectiveness by up to 30% while lowering expenses. However, researchers must remain vigilant about the risks, such as potential bias from mid-study changes and regulatory skepticism. As Lorraine Hazlehurst observed, 'Collaborations across fields are vital to address these challenges, especially regarding privacy, technology access, and the need for consistent terminology in the context of digital interfaces.'
This multifaceted approach underscores the necessity for meticulous planning and strategic consideration throughout the design phase to yield reliable and valid results, which aligns with the primary endpoint meaning of robust project management and monitoring. A case study on adaptive study designs illustrates these points: while they offer advantages such as improved endpoint sensitivity and shorter study durations, they also pose challenges including potential bias and complex management requirements. Furthermore, medtech research studies have a substantial effect on local economies, aiding in job creation and healthcare enhancement, which further highlights the significance of these experiments.
Comprehending the regulatory environment is also important; INVIMA, as Colombia's national food and drug monitoring agency, plays a critical role in supervising medical devices and ensuring adherence to health regulations, which is necessary for the successful implementation of trials.
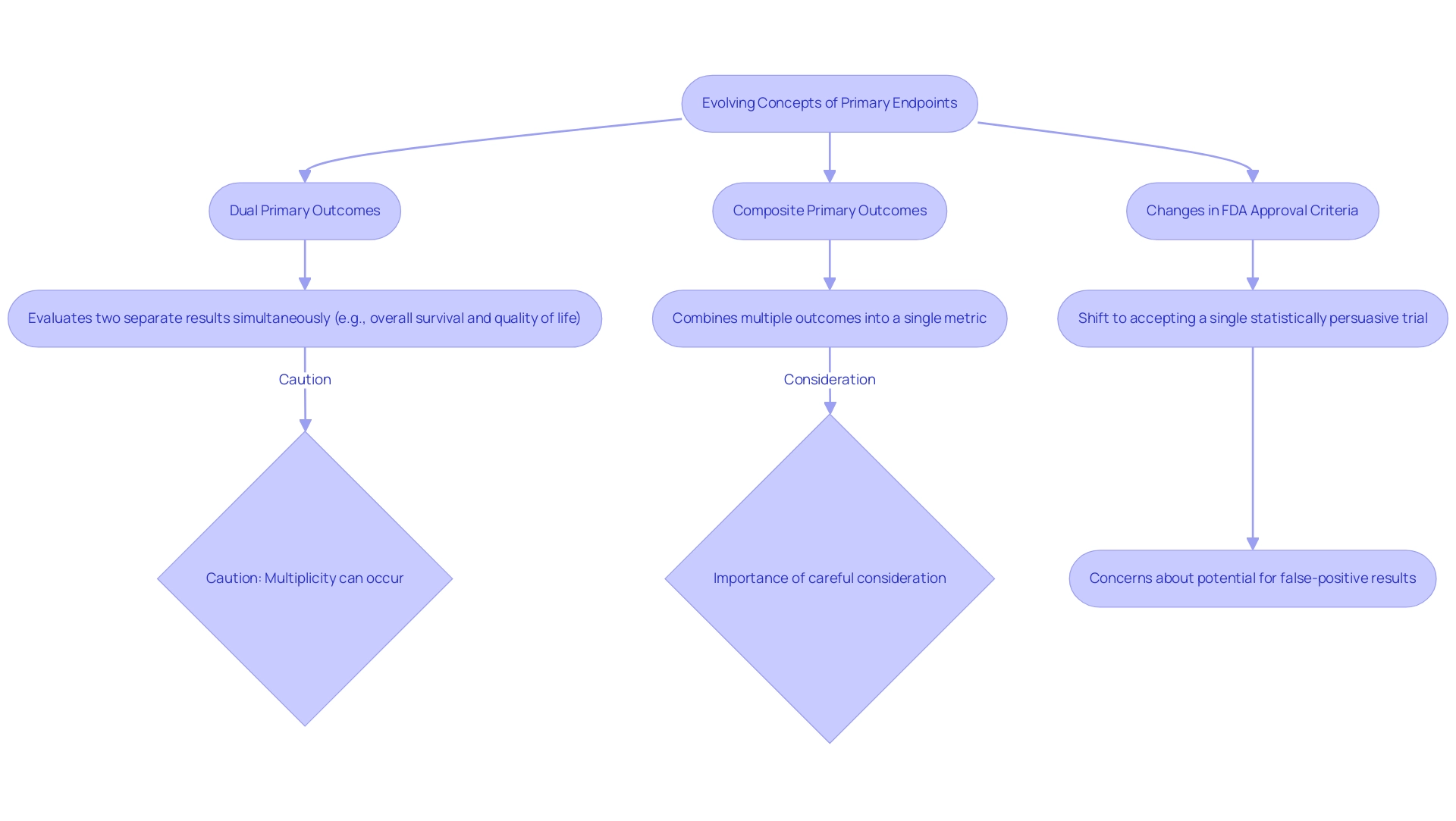
Evolving Concepts: Dual and Composite Primary Endpoints
The increasing interest in dual and composite primary outcomes reflects their potential to offer a more nuanced assessment of treatment effects, which is crucial for understanding primary endpoint meaning in medical research. Two primary objectives involve evaluating two separate results simultaneously, which improves the comprehension of a treatment's effectiveness. For example, a clinical trial may evaluate both overall survival and quality of life, allowing for a holistic view of patient outcomes.
In contrast, composite primary measures amalgamate multiple individual outcomes into a single metric. This methodology not only captures a broader spectrum of treatment effects but also enhances the primary endpoint meaning while minimizing the sample size needed for the study and increasing statistical power. A significant example is the demonstration of the superiority of coronary artery bypass grafting (CABG) over percutaneous coronary intervention (PCI) when including target vessel revascularization (TVR) in the composite, showcasing the effectiveness of composite measures.
However, as John Lawrence, a statistician at the FDA’s Center for Drug Evaluation and Research, cautions:
Multiplicity can occur because there is a chance of making a mistake during the evaluation of each target in the study, and the chances of making a mistake can accumulate when assessing multiple targets if the appropriate statistical adjustments are not made.
Thus, careful consideration is paramount to ensure that the chosen composite or dual measures preserve their clinical relevance and align with the primary endpoint meaning. Recent trends in medical research emphasize the necessity for a meticulous approach, as the FDA's shift towards accepting a single statistically convincing study for drug approvals raises concerns regarding the potential for false-positive results.
This change, which has led to accelerated approvals based on single trials, underscores the importance of adequately defining and justifying the use of dual and composite measures, ensuring they contribute meaningfully to the clinical decision-making process. Additionally, the tutorial by Amorim and Cai on modeling recurrent events in epidemiology provides valuable insights into the statistical methodologies relevant to these endpoints, enriching the discussion on their practical implications.
Conclusion
The selection of primary endpoints is undeniably a critical aspect of clinical trial design, influencing not only the statistical outcomes but also the broader implications for patient care and regulatory approval. Throughout this discussion, the importance of defining primary endpoints was emphasized, highlighting their role in assessing treatment efficacy and ensuring that trials yield meaningful data. The distinction between clinical, surrogate, composite, and time-to-event endpoints illustrates the complexity involved in endpoint selection, with each type serving specific research objectives and patient needs.
Moreover, the article addressed the challenges faced in defining and measuring these endpoints, particularly the necessity for clarity and relevance amidst evolving methodologies in clinical research. The introduction of dual and composite primary endpoints represents a significant advancement, allowing for a more comprehensive evaluation of treatment effects while also raising concerns about statistical multiplicity. As the landscape of clinical trials continues to adapt, the need for meticulous planning and strategic consideration in endpoint selection becomes increasingly apparent.
In conclusion, the careful definition and measurement of primary endpoints are vital for the integrity and success of clinical trials. Stakeholders must remain vigilant in their approach, ensuring that endpoints align with trial objectives and contribute meaningfully to clinical decision-making. As medical research progresses, fostering a deeper understanding of endpoint dynamics will not only enhance the credibility of trial results but also facilitate the advancement of medical knowledge and improve patient outcomes.
Frequently Asked Questions
What are main objectives in medical research?
Main objectives are crucial results that a research study is designed to assess, serving as the basis for evaluating the effectiveness of therapies or interventions.
Why is the choice of a primary endpoint important?
The choice of a primary endpoint is important because it directly influences the study's design, statistical evaluation, and the interpretation of findings.
What are some examples of main outcomes in research studies?
Examples of main outcomes include overall survival rates, disease progression-free survival, and relief of symptoms in patients.
How does the U.S. FDA view the selection of study objectives?
The U.S. FDA emphasizes that the selection of objectives must be guided by scientific evidence and relevance to patient care, as stated in its 2023 guidance on decentralized studies.
What are the classifications of main objectives in medical research?
Main objectives can be classified into clinical targets, surrogate measures, composite measures, and time-to-event endpoints.
What are clinical targets?
Clinical targets are measures directly associated with patient results, such as survival rates and health-related quality of life, providing clear evidence of a treatment's effectiveness.
What are surrogate measures?
Surrogate measures are indirect indicators expected to predict clinical benefits but do not directly reflect patient outcomes, such as laboratory results or imaging findings.
What are composite measures?
Composite measures include several individual measures, allowing researchers to capture a wider range of treatment effects by merging different results.
What are time-to-event endpoints?
Time-to-event endpoints measure the duration until a specific event occurs, such as disease recurrence or patient death, and are particularly useful in chronic illness research.
How does the current trend in medical research affect the use of substitute measures?
The use of substitute measures is becoming more common, especially with the growth of digital health technologies and connected devices in studies.
Why is understanding the intricacies of primary endpoints vital?
Understanding these intricacies is vital for maintaining the integrity of research outcomes and ensuring accurate findings.




