Overview
The best contract research organization (CRO) plays a vital role in clinical trials by providing specialized services such as regulatory compliance, patient recruitment, and data management, which enhance the efficiency and effectiveness of drug development. The article supports this by illustrating how leading CROs like ICON and bioaccess® leverage their expertise and advanced technologies to navigate complex regulatory landscapes and improve trial outcomes, ultimately facilitating faster access to innovative therapies.
Introduction
The evolving landscape of clinical research is increasingly shaped by the pivotal role of Contract Research Organizations (CROs), which serve as essential partners in the drug development process. As specialized entities, CROs provide a comprehensive suite of services that streamline the complexities of clinical trials, from regulatory compliance to patient recruitment and data management.
With the rising demand for innovative therapies and the growing intricacies of regulatory environments, the collaboration between pharmaceutical companies and CROs is more crucial than ever. This article delves into the significance of CROs, the criteria for selecting the right partner, the diverse services they offer, the transformative impact of technology, and the emerging trends that are set to redefine the future of clinical research.
Through an exploration of these key aspects, a clearer understanding of how CROs can enhance trial efficiency and drive successful outcomes in the ever-competitive medical landscape will be established.
Understanding Contract Research Organizations: Definition and Importance
The best contract research organization functions as a specialized entity that provides comprehensive services crucial for the planning, execution, and management of clinical studies. As the best contract research organization, their role in the drug development process is invaluable, providing deep expertise in regulatory compliance, patient recruitment, data management, and monitoring. For medical device startups, navigating regulatory hurdles and competition can be daunting; however, contract research organizations streamline these processes by providing comprehensive services including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting on study status
- Feedback on study documents
By outsourcing these essential investigative activities to CROss, pharmaceutical firms and research organizations can utilize specialized skills and resources that improve both efficiency and effectiveness in study results. For example, ICON has successfully carried out over 660 heart studies involving more than 710,000 patients in the past five years, demonstrating their ability to lead substantial medical investigation initiatives. Moreover, recent industry developments highlight that 7.2% of trials with a decentralized clinical trial (DCT) component focus specifically on oncology, underscoring the evolving landscape of clinical trials.
As highlighted by Syneos Health, 'we are at the forefront of innovative cardiovascular studies and patient-centered care,' which underscores the vital role contract research organizations play in promoting medical advancement. The significance of the best contract research organization goes beyond simple assistance; they are crucial in tackling the difficulties encountered by medical device startups by optimizing the development process, lowering operational expenses, and speeding up the time to market for new treatments. For example, Precision for Medicine's recent expansion, following their acquisition of Baseline Controls in 2023, enhances their biomanufacturing strategy, driving efficiency in drug development.
Furthermore, recent news regarding Proclinical seeking a Senior Principal Biostatistician emphasizes ongoing advancements in the CRO sector, ultimately improving patient access to innovative therapies and reinforcing CROss as foundations of contemporary research. To explore how our services can assist you in advancing your medical device evaluations sooner, BOOK A MEETING.
Choosing the Right CRO: Key Criteria and Considerations
Selecting the best contract research organization (CRO) involves careful consideration of several essential criteria. First and foremost, stakeholders should prioritize a CRO's expertise in the relevant therapeutic area, as this knowledge is essential for navigating the complexities of research studies. A strong track record of successful studies is equally important; organizations should seek the best contract research organization that has demonstrable success rates aligned with their specific research objectives.
For instance, bioaccess® has over 20 years of experience in managing medical device research studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
making them a strong candidate for CRO selection.
Additionally, bioaccess® is recognized for its adaptability and expert knowledge, which are essential in adjusting to the distinct requirements of each clinical study. The capability to offer customized services that satisfy specific project needs can greatly improve the chances of success. Effective communication practices and cultural fit between the CRO and the study team are crucial for fostering collaboration and ensuring smooth project execution.
Evaluating the CRO's capacity to manage timelines and budgets is imperative, as these factors directly impact trial efficiency and outcomes. As technology continues to evolve, assessing a CRO's technological capabilities becomes increasingly essential. Advanced data management systems and innovative tools can streamline processes, leading to improved operational efficiency and data integrity.
Incorporating insights from industry leaders, such as Elizabeth Romanski, who emphasizes that 'you don't need a website development background to make VWO work for you. The VWO support team is amazing,' highlights the importance of support and usability in CRO services. Conducting thorough due diligence, including seeking references from previous clients, is a critical step in the selection process.
This approach not only provides insights into the CRO's performance but also ensures alignment with the research goals and organizational values. Additionally, considering regulatory functions and oversight from entities like INVIMA, which classifies medical devices in Colombia and plays a significant role in ensuring local compliance, underscores the necessity for the best contract research organization to adapt their services to meet these needs. By thoughtfully considering these elements, stakeholders can make knowledgeable choices that improve the success of their research studies.
Services Offered by CROs: A Comprehensive Overview
The best contract research organization (CRO) offers a varied range of services that are essential for the efficient oversight of research studies. These services can be categorized into several key areas:
- Study design and protocol development
- Site selection and management
- Patient recruitment and retention
- Data management and statistical analysis
- Regulatory Affairs support, including detailed review and feedback on study documents to ensure compliance with country requirements.
Among the leaders in this field is bioaccess®, which specializes in medical device studies in Latin America, bringing over 20 years of expertise to the forefront.
Their comprehensive service capabilities encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Meticulous reporting on study status and adverse events
This comprehensive suite enables sponsors to maneuver through the complex environment of research studies effectively, ensuring that investigations are conducted in accordance with regulatory standards. As the quantity of registered medical studies globally is expected to rise considerably in 2024, the specialized assistance offered by bioaccess® becomes more crucial for the successful implementation of trials.
Their focus on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) exemplifies their commitment to innovation and regulatory excellence. As pointed out by research specialist Matej Mikulic, 'Contact us now to discover how the best contract research organization can assist with your study requirements.
The Role of Technology in Modern CROs: Enhancing Clinical Trials
In the modern environment of the best contract research organization (CRO), technology is not just a supplementary element; it is a cornerstone that greatly improves the efficiency and effectiveness of clinical studies. Our service capabilities include:
- Feasibility studies
- Meticulous site selection
- Comprehensive compliance reviews and setup, which are crucial for ensuring regulatory adherence
- Handling import permits
- Providing detailed reporting on study status, inventory, and both serious and non-serious adverse events
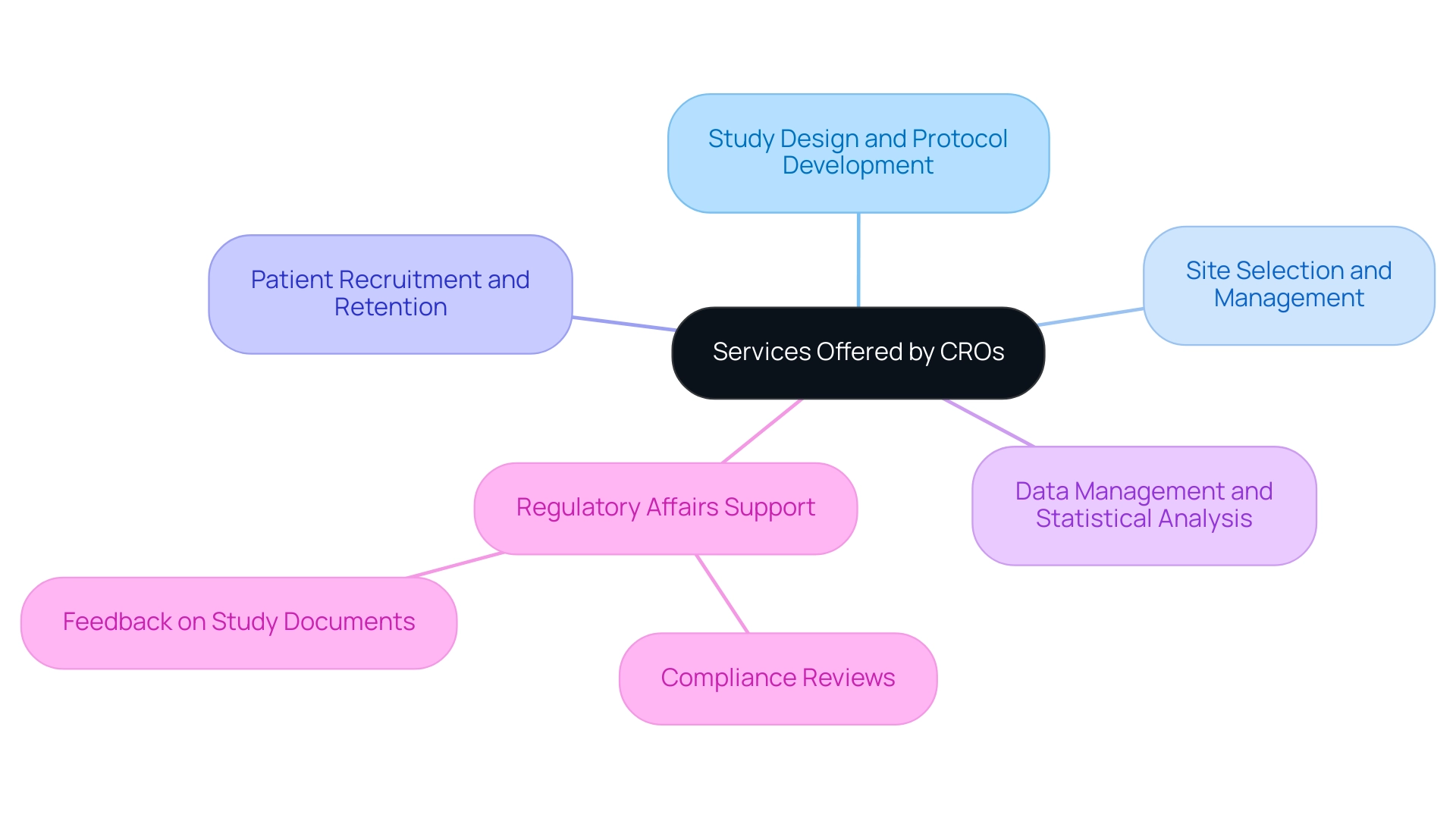
For instance, the success rates in Phase I studies have surged to 48%, while Phase III success rates have risen to an impressive 66%, surpassing the pre-pandemic average of 56%. This remarkable improvement underscores the critical role of advanced data management systems and electronic data capture (EDC) solutions in facilitating real-time data collection and analysis. These technologies not only streamline regulatory compliance processes but also foster greater patient engagement through digital platforms.
As Colin Weller, General Manager of Digital Endpoints and Measures, aptly states, 'Given this and an increasing number of validated outcomes that can be measured by wearables, digital tools will play a crucial role in breaking down geographical and logistical barriers, offering broader access flexibility for patients in clinical studies.' Additionally, the incorporation of artificial intelligence and machine learning in patient recruitment and data analysis is becoming more common, enabling CROs to optimize study designs and enhance outcomes. For example, AI algorithms can analyze vast datasets to identify suitable candidates for experiments more efficiently, significantly reducing recruitment times.
The trend toward decentralized endpoint strategies reflects a commitment to improving patient access and diversifying trial participation, as seen in the growing interest in psychedelics for treating CNS disorders, with nearly 200 studies currently registered. This marks a significant step forward in the evolution of clinical research and highlights the impact of Medtech clinical studies on job creation, economic growth, and healthcare improvement. As the industry continues to adapt, the adoption of these technological advancements will be essential for the best contract research organization to sustain their competitiveness and drive global health improvement through international collaboration and innovation.
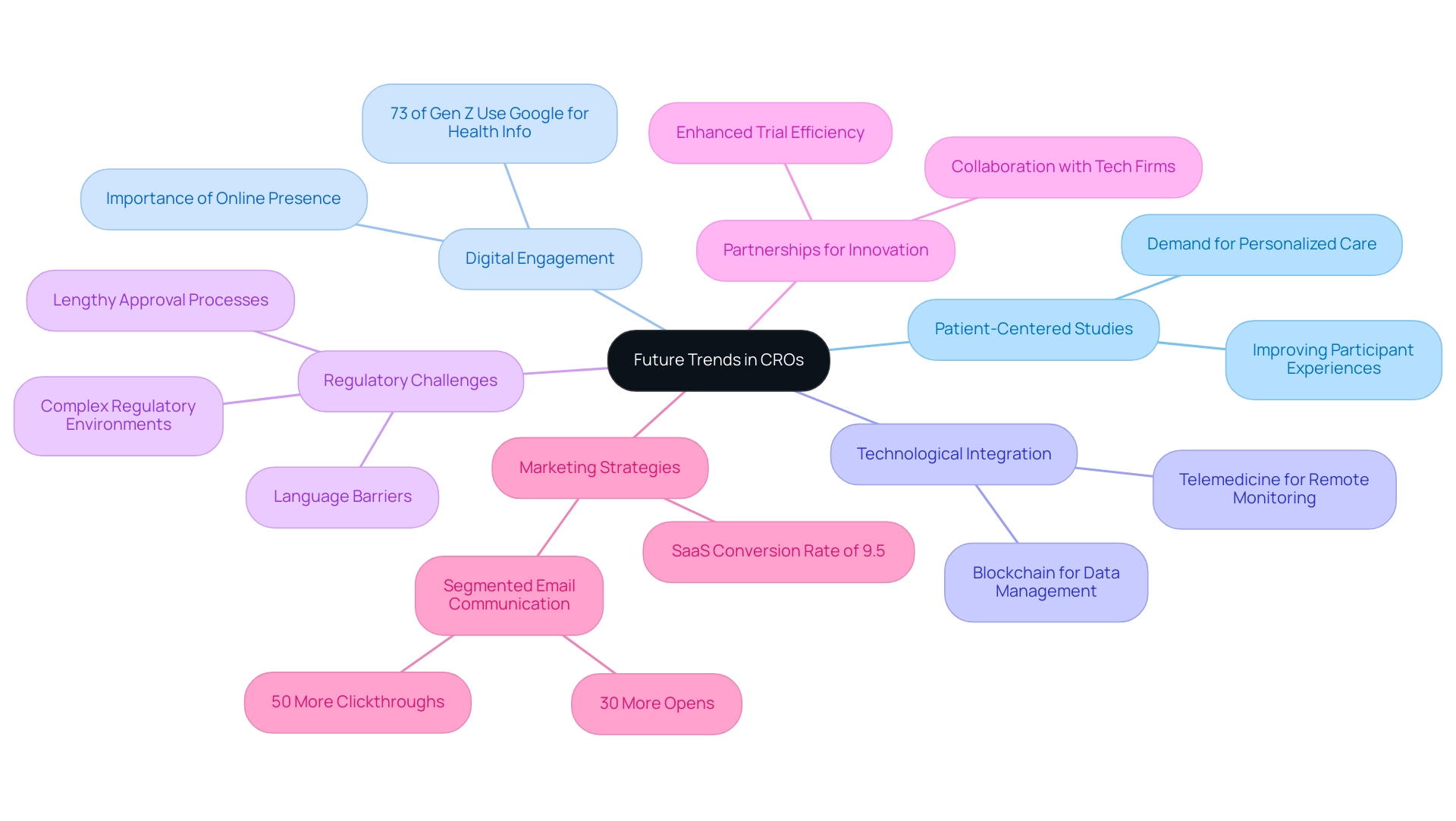
Future Trends in Contract Research Organizations: What Lies Ahead
The landscape of the best contract research organization (CRO) is on the brink of significant transformation, driven by several pivotal trends. A primary emphasis is the growing dedication to patient-centered studies, which seeks to improve participant experiences throughout trials. This shift is crucial, as studies indicate a growing demand for more personalized care approaches in clinical settings.
Notably, 73% of Gen Z turn to Google before consulting a medical professional, underscoring the importance of digital engagement in patient-centric research. Furthermore, the integration of advanced technologies—such as blockchain for secure data management and telemedicine for remote patient monitoring—will revolutionize trial methodologies, making them more efficient and accessible. As regulatory environments become increasingly complex, particularly in Latin America, clinical research organizations must bolster their compliance capabilities and expand their expertise in navigating these challenges, including specific regulatory hurdles like lengthy approval processes and language barriers that can hinder effective communication.
This adaptability is essential for maintaining integrity and trust in research outcomes. Additionally, the trend of forming partnerships between the best contract research organizations and technology firms is set to yield innovative solutions that significantly enhance trial efficiency and data integrity, facilitating smoother collaboration between American clinical trial clients and Latin American hospitals. For instance, the best contract research organization can provide comprehensive services such as feasibility studies, site selection, and compliance reviews, which are critical for navigating the regulatory landscape.
The SaaS industry, for example, has an average conversion rate of 9.5%, emphasizing the importance of effective marketing strategies in conversion rate optimization. Moreover, case studies show that segmented email communication can drive 30% more opens and 50% more clickthroughs than unsegmented ones, illustrating how tailored engagement can improve participant experiences. By proactively embracing these trends and addressing the unique challenges in Latin America, including the need for robust support structures and local expertise, CROss can solidify their positions as leaders in the evolving clinical research arena, ensuring they meet the rising expectations of stakeholders and participants alike.
Conclusion
The role of Contract Research Organizations (CROs) in the clinical research landscape is integral to the efficient development of new therapies. As highlighted throughout the article, CROs provide a comprehensive suite of services that encompass everything from regulatory compliance to patient recruitment and data management. Their expertise not only streamlines the complexities of clinical trials but also significantly enhances the likelihood of successful outcomes. The importance of selecting the right CRO cannot be overstated, as factors such as therapeutic expertise, a proven track record, and adaptability are crucial in ensuring that trials meet their objectives.
Furthermore, the incorporation of advanced technology into CRO operations is revolutionizing the way clinical trials are conducted. The integration of digital tools and artificial intelligence not only improves operational efficiency but also fosters greater patient engagement, making trials more accessible to diverse populations. As the landscape continues to evolve, emerging trends such as patient-centric research and partnerships with technology firms are set to redefine the future of CROs, enhancing their capabilities to navigate complex regulatory environments and meet the demands of an increasingly informed patient population.
In conclusion, the collaboration between pharmaceutical companies and CROs is more critical than ever in the quest for innovative therapies. By leveraging the specialized skills and resources that CROs offer, stakeholders can navigate the intricacies of clinical research with greater confidence and efficiency. As the industry adapts to new challenges and opportunities, CROs will remain at the forefront of medical advancement, driving innovation and improving patient outcomes in the ever-competitive healthcare landscape.
Frequently Asked Questions
What is the role of a contract research organization (CRO) in clinical studies?
A contract research organization (CRO) provides comprehensive services essential for the planning, execution, and management of clinical studies, including expertise in regulatory compliance, patient recruitment, data management, and monitoring.
What services do the best contract research organizations offer?
The best CROs offer a range of services including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, reporting on study status, and feedback on study documents.
How do CROs benefit pharmaceutical firms and research organizations?
By outsourcing investigative activities to CROs, pharmaceutical firms and research organizations can leverage specialized skills and resources, improving efficiency and effectiveness in study results.
Can you provide an example of a successful CRO?
ICON is an example of a successful CRO, having conducted over 660 heart studies involving more than 710,000 patients in the past five years.
What recent trends are observed in clinical trials involving CROs?
Recent trends indicate that 7.2% of trials with a decentralized clinical trial (DCT) component focus specifically on oncology, reflecting the evolving landscape of clinical trials.
What factors should stakeholders consider when selecting a CRO?
Stakeholders should consider a CRO's expertise in the relevant therapeutic area, a strong track record of successful studies, adaptability, effective communication practices, capacity to manage timelines and budgets, and technological capabilities.
What specific experience does bioaccess® have in medical device research?
Bioaccess® has over 20 years of experience managing medical device research studies, including early-feasibility studies, first-in-human studies, pilot studies, pivotal studies, and post-market follow-up studies.
Why is effective communication important in CRO selection?
Effective communication practices and cultural fit between the CRO and the study team are crucial for fostering collaboration and ensuring smooth project execution.
How can stakeholders ensure they choose the right CRO?
Conducting thorough due diligence, including seeking references from previous clients, is essential to gain insights into the CRO's performance and ensure alignment with research goals and organizational values.
What regulatory considerations should be taken into account when selecting a CRO?
Stakeholders should consider the CRO's ability to adapt services to meet regulatory functions and oversight requirements, such as those from entities like INVIMA, which classifies medical devices in Colombia.

