Overview
Latin America is an ideal location for medical trials due to its cost efficiency, diverse patient population, and improving regulatory landscape, which collectively enhance the speed and quality of clinical research. The article highlights that significant savings in labor and facility costs, coupled with a commitment to ethical standards and a rich ethnic diversity among participants, create a conducive environment for effective and inclusive medical studies.
Introduction
Latin America has emerged as a pivotal player in the realm of clinical trials, driven by a confluence of strategic advantages that appeal to researchers and sponsors alike. The region's unique blend of cost efficiency, patient accessibility, and a commitment to ethical standards creates an environment ripe for innovation in clinical research.
Recent collaborations, such as those between bioaccess™ and Caribbean Health Group, underscore the ongoing efforts to enhance infrastructure and capabilities in key locations like Barranquilla, positioning Latin America as a prime destination for conducting trials.
As the global landscape of clinical research evolves, understanding the factors that contribute to the region's growing significance is essential for stakeholders aiming to leverage its potential.
This article delves into the multifaceted benefits and challenges of conducting clinical trials in Latin America, shedding light on the regulatory landscape, the diversity of patient populations, and the strategic initiatives that are shaping the future of clinical research in this dynamic region.
The Strategic Advantages of Latin America for Clinical Trials
The region has strategically placed itself as a center for research studies, prompting the question of why Latin America for medical trials, by offering a distinctive combination of benefits that draw in researchers and sponsors alike. On March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, the collaboration between bioaccess™ and Caribbean Health Group was announced, aiming to position Barranquilla as a leading destination for clinical studies in Latin America, receiving support from Colombia's Minister of Health. This collaboration aims to boost the region's attractiveness by enhancing the infrastructure and abilities essential for successful assessments.
Moreover, GlobalCare Clinical Trials' partnership with bioaccess™ has led to significant advancements in ambulatory services, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate. The area's geographical closeness to the United States improves collaboration and logistics, ultimately increasing efficiency. As regional experts acquire knowledge in medical research, the overall standard of studies carried out in the southern continent continues to enhance.
The diverse cultural landscape, paired with a willingness among local populations to participate, creates a favorable environment for research. However, ethical considerations must be addressed, as patients may agree to participation without thorough discussions about treatment options. In 2023, the region represented 2.1% of the worldwide research market, highlighting why Latin America for medical trials is a focus, as this number is anticipated to grow with global organizations acknowledging its potential.
bioaccess® specializes in managing various types of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
As Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, states, 'Having a local presence and leadership based in the LATAM region strengthens the relationships that Parexel builds with local talent, regulatory authorities, and the global network of sites overall.' This synergy between local engagement and global collaboration, along with the expected growth in the market, strongly positions the region as an appealing option, prompting the question: why Latin America for medical trials?
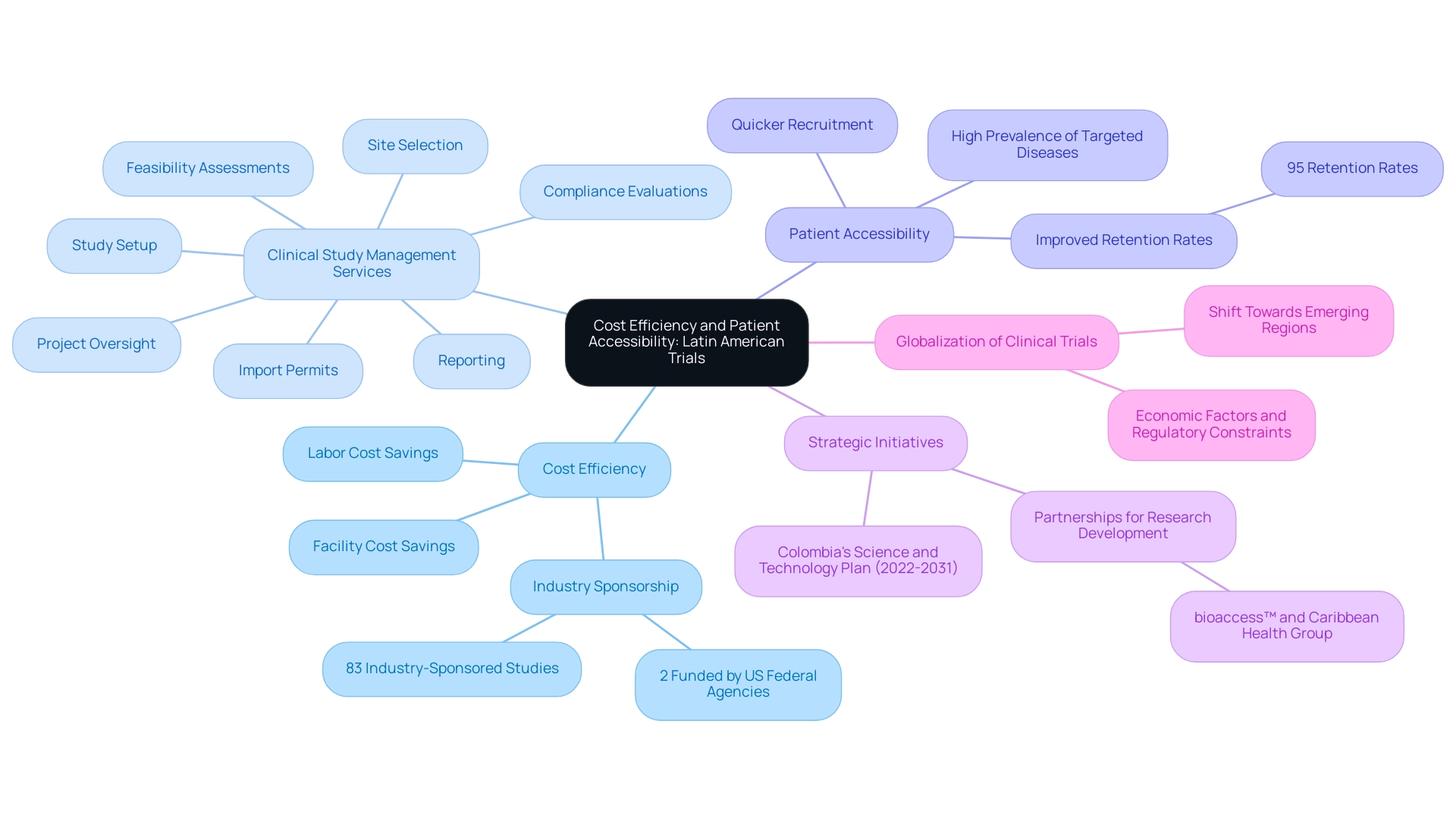
Cost Efficiency and Patient Accessibility: Key Benefits of Latin American Trials
The significant benefits of carrying out clinical studies in South regions raise the question: Why Latin America for medical trials, particularly in terms of cost efficiency and a wide range of clinical study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
The significant labor and facility cost savings compared to the United States raise the question of why Latin America for medical trials, as an average of 83% of studies in the region are industry-sponsored, highlighting the appeal for pharmaceutical companies seeking cost-effective solutions. The high prevalence of targeted diseases in the region raises the question of why Latin America for medical trials, as it improves patient accessibility and facilitates quicker recruitment and retention of participants.
This is particularly crucial, as understanding why Latin America for medical trials is important can lead to efficient patient recruitment strategies that dramatically influence trial timelines and outcomes. Significantly, only 2% of studies in the region are funded by the National Institutes of Health and other US Federal Agencies, highlighting the dependence on industry sponsorship. Colombia has acknowledged these advantages and is actively pursuing an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy, which prompts the inquiry of why Latin America for medical trials, demonstrating a commitment to fostering an environment conducive to research.
Furthermore, the partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, seeks to establish Barranquilla as a prominent location for medical studies, highlighting why Latin America for medical trials. This initiative is not only anticipated to boost the local economy through job creation and healthcare enhancement but also to optimize clinical study ambulatory services, leading to over a 50% reduction in recruitment time and 95% retention rates. The anticipated results concerning study project management and monitoring will guarantee that experiments are carried out efficiently and effectively.
The case study titled 'Current Globalization of Drug Interventional Clinical Studies' further emphasizes why Latin America for medical trials is strategically beneficial. By optimizing resource distribution through these dual benefits of cost and accessibility, studies in South America illustrate why Latin America for medical trials is advantageous, as they not only speed up research timelines but also lead to more effective and reliable research outcomes.
Navigating the Regulatory Landscape: Support for Clinical Trials in Latin America
The considerable development of the regulatory landscape in Latin America raises the question of why Latin America for medical trials, as various nations actively enact reforms to accelerate the research approval process. This supportive environment is characterized by a strong dedication to ethical standards and compliance, ensuring that all studies are conducted with the utmost responsibility. In Colombia, our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Handling of import permits
All aimed at simplifying the process for sponsors.
The setup process involves obtaining necessary approvals from ethics committees and the health ministry, which are crucial for ensuring that the studies meet local regulations. With a focus on project management and detailed reporting, we ensure that clinical research adheres to local regulations while optimizing efficiency. Colombia checks positively on several competitive advantages for first-in-human studies:
- Cost efficiency, with savings of over 30% compared to North America and Western Europe
- Regulatory speed, with IRB/EC and INVIMA approval typically taking just 90-120 days
- A high-quality healthcare system ranked among the best in the area
The strong patient enrollment, supported by a population exceeding 50 million and comprehensive healthcare coverage, along with R&D tax incentives—such as a 100% tax deduction on investments in science, technology, and innovation—raises the question of why Latin America for medical trials. As regulatory experts emphasize, these favorable conditions are essential for fostering innovation and ensuring patient safety, ultimately paving the way for more effective therapies to reach the patient population.
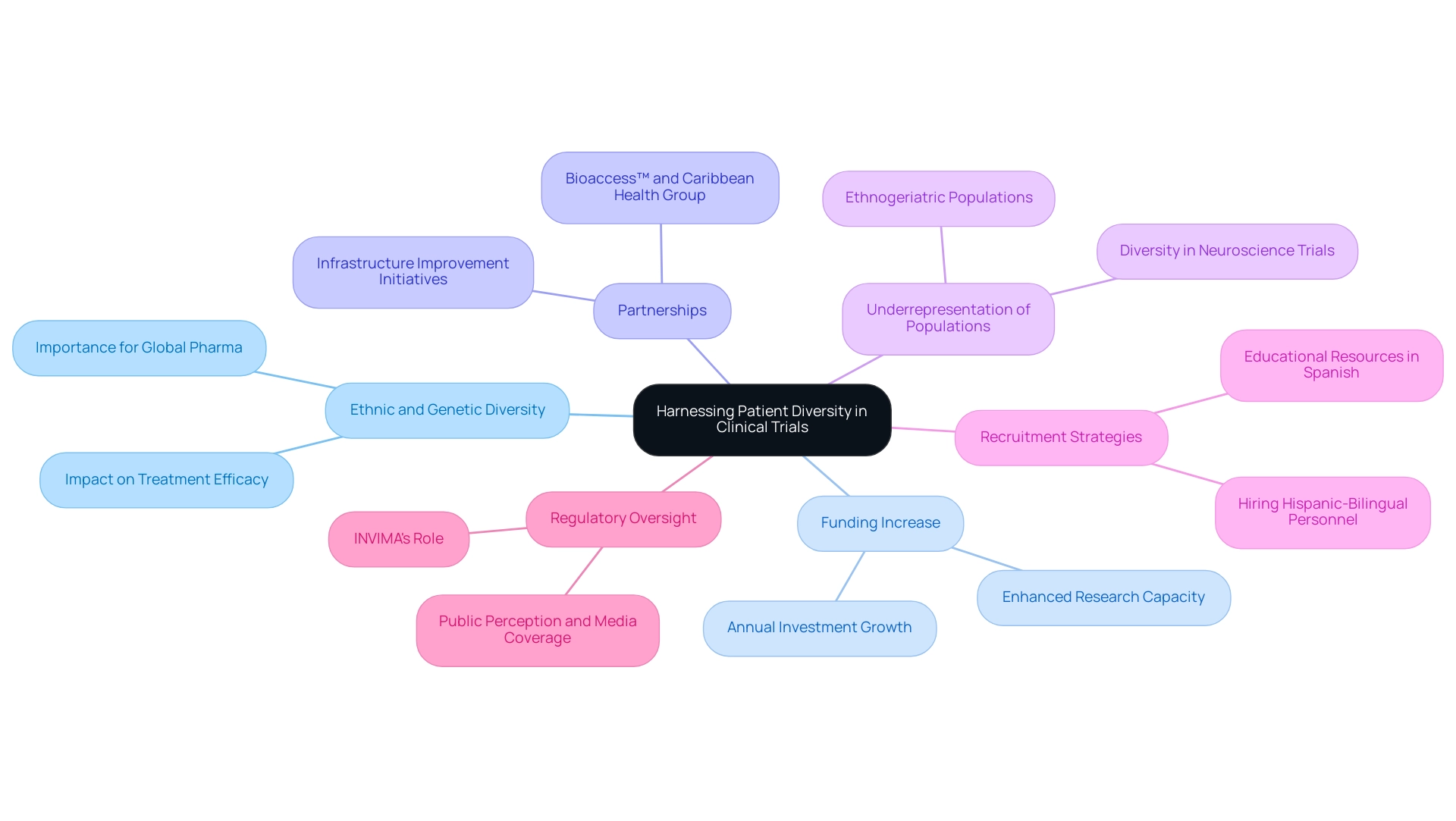
Harnessing Patient Diversity: Enhancing Clinical Trial Outcomes
The question of why Latin America for medical trials is significant is highlighted by its abundant ethnic and genetic variety, which offers a distinctive chance for studies, enabling researchers to investigate how different populations react to treatments. This diversity is crucial for enhancing the validity of study outcomes, and it raises the question of why Latin America for medical trials can provide a broader understanding of the efficacy and safety of new therapies. By involving a diverse group of participants, research studies can produce extensive data, resulting in more informed conclusions.
This aspect is particularly vital for global pharmaceutical companies that aim to explore why Latin America for medical trials is important in developing treatments effective across diverse demographics. The yearly funding in the research sector in the Andean Region has significantly increased from $3-4 million to over $50 million, indicating a growing acknowledgment of these benefits. This increased investment not only enhances the capacity for conducting diverse studies but also highlights why Latin America for medical trials is crucial to ensure that new therapies are safe and effective for all demographics.
Moreover, the partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, seeks to establish Barranquilla as a prominent location for medical studies, highlighting the question of why Latin America for medical trials while improving the region's infrastructure for research. Experts such as VJ Periyakoil highlight that addressing the underrepresentation of ethnogeriatric populations—those aged 65 and older—is essential, raising the important question of why Latin America for medical trials, as these groups are still significantly lacking in research studies. A retrospective review of the F. Hoffmann-La Roche database, titled 'Diversity in Neuroscience Trials,' revealed that 85.6% of participants were White, showcasing the significant underrepresentation of Black individuals and Hispanic or Latino participants compared to national population data.
Implementing effective recruitment strategies, such as offering educational resources in Spanish and hiring Hispanic-bilingual personnel, can help illustrate why Latin America for medical trials is important in reducing unconscious biases and improving the involvement of Hispanic and Latino individuals in studies. These strategies directly address the key point of improving diversity by fostering an inclusive environment that encourages participation. Such efforts not only encourage fairness in research but also contribute to understanding why Latin America for medical trials is significant, as they reveal how varied patient populations react to treatments, ultimately enhancing study results.
Moreover, INVIMA's function as the regulatory body supervising research studies in Colombia is crucial in ensuring adherence and safety, further boosting the credibility of the studies carried out in the area. Media coverage of these proceedings, as reported by Clinical Leader, plays a significant role in shaping public perception and awareness, thereby attracting more interest and investment in research in Colombia.
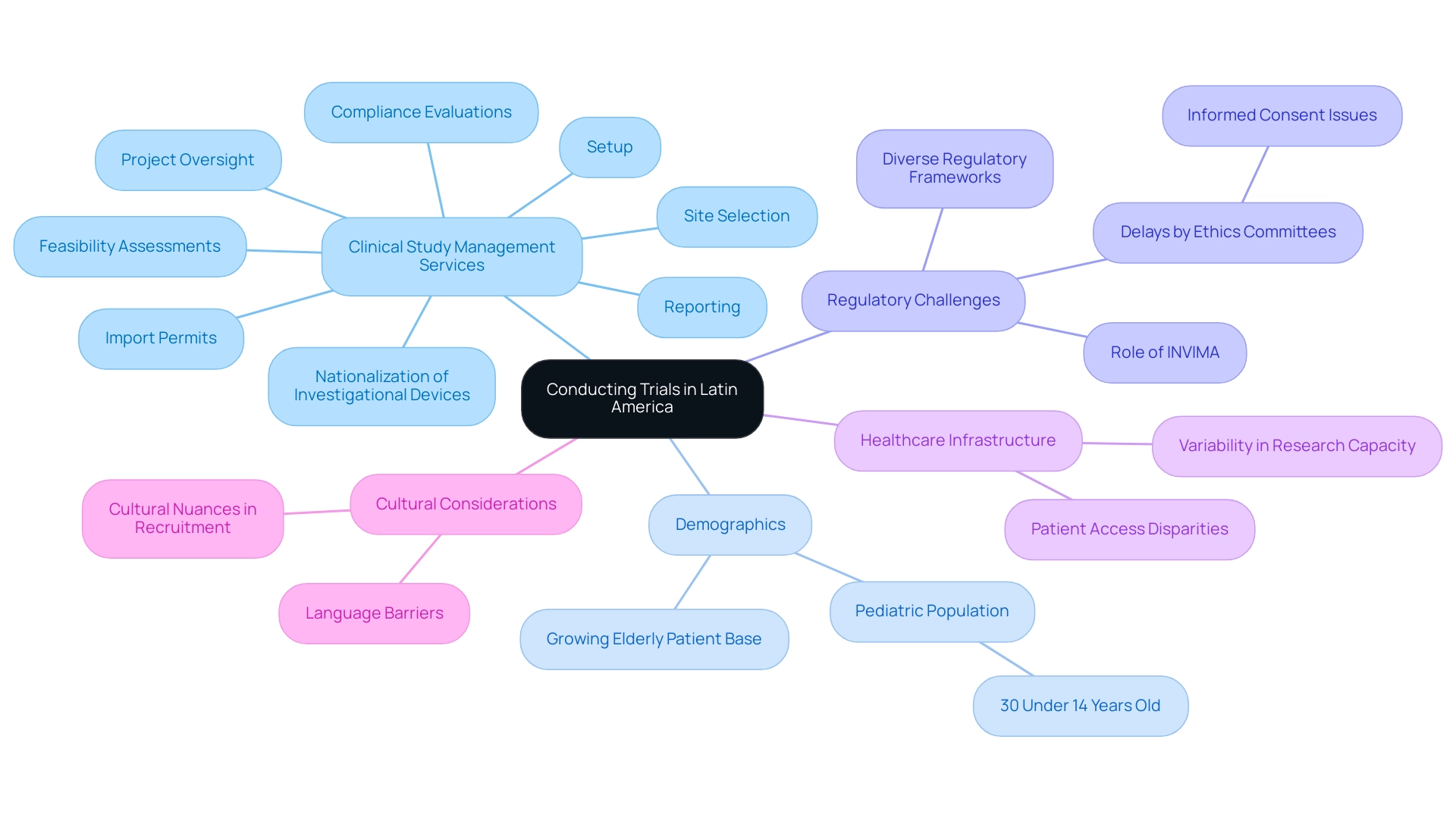
Challenges and Considerations: Conducting Trials in Latin America
When considering the region's multifaceted landscape for clinical studies, one might ask why Latin America for medical trials, as it offers significant benefits through extensive clinical study management services that include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Nationalization of investigational devices
- Project oversight
- Reporting
While the region's healthcare infrastructure showcases variability, leading to disparities in research capacity and patient access, it also features a substantial pediatric population, with 30% of individuals under 14 years old. This demographic signals not only a growing elderly patient base but also an expanding market for potential drug development.
Additionally, data from 2009–2010 revealed that only 6.1% of medical oncologists in Latin America and the Caribbean were the main sponsors of their research proposals, highlighting a critical area for local research leadership growth. Researchers need to navigate language barriers and diverse regulatory frameworks, which can vary significantly across countries. Cultural nuances further complicate patient recruitment and retention strategies, necessitating a tailored approach to trial design.
Delays by ethics committees, often linked to issues with informed consent, can be effectively mitigated by addressing these linguistic and cultural differences. Colombia exemplifies a proactive stance in this regard, having recognized the benefits of medical research through its ambitious science, technology, and innovation plan for 2022–2031, aiming to transition into a knowledge economy. This local expertise, combined with thorough due diligence, allows sponsors to fully leverage the region's unique opportunities while addressing inherent risks.
Moreover, the steady rise in scientific publications related to oncology in the southern continent—from 1,978 documents in 2015 to 3,410 in 2020—reflects the potential for growth, although the region's scientific productivity remains significantly lower than that of Northern America and Western Europe. By considering these factors, including the role of INVIMA as a regulatory authority overseeing medical devices and drug approvals, and staying informed of regulatory insights, we can explore why Latin America for medical trials can optimize clinical research, paving the way for more successful and inclusive studies. Reporting aspects such as study status, inventory management, and serious and non-serious adverse events must also be integrated into the trial management process to enhance compliance and transparency.
Conclusion
Latin America has emerged as a key player in clinical trials, offering unique advantages that attract researchers and sponsors. Strategic partnerships, such as that between bioaccess™ and Caribbean Health Group, enhance local infrastructure in cities like Barranquilla. The region's cost efficiency and patient accessibility are significant, with a majority of studies being industry-sponsored, which accelerates participant recruitment and retention.
The regulatory landscape has evolved to support clinical trials through streamlined approval processes and a strong commitment to ethical standards. Countries like Colombia exemplify this shift, creating a favorable environment for first-in-human trials while ensuring compliance and safety. Additionally, the diverse patient populations in Latin America enrich research, providing a broader understanding of treatment efficacy across demographics.
While challenges such as regulatory variability and cultural nuances exist, addressing these through tailored recruitment strategies and local expertise is essential for maximizing trial potential. The increasing investment in the sector highlights the importance of inclusivity and diversity in trial populations for developing effective therapies.
In summary, Latin America's combination of cost efficiency, supportive regulatory frameworks, and diverse patient demographics positions it as an emerging leader in clinical trials. Stakeholders must navigate challenges while leveraging the region's strengths to foster innovation, ultimately making significant contributions to the global clinical research landscape.
Frequently Asked Questions
Why is Latin America considered a favorable location for medical trials?
Latin America offers a unique combination of benefits, including cost efficiency, a diverse cultural landscape, and a willingness among local populations to participate, making it an attractive option for researchers and sponsors.
What initiatives are being taken to enhance Barranquilla as a destination for clinical studies?
A collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, aims to position Barranquilla as a leading destination for clinical studies by improving infrastructure and capabilities for successful assessments.
How have partnerships in the region improved clinical trial processes?
The partnership between GlobalCare Clinical Trials and bioaccess™ has led to significant advancements, including over a 50% reduction in recruitment time and a 95% retention rate for participants.
What role does geographical proximity to the United States play in medical trials in Latin America?
The region's geographical closeness to the United States enhances collaboration and logistics, leading to increased efficiency in conducting clinical trials.
What types of studies does bioaccess® manage?
bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
What are the ethical considerations involved in conducting medical trials in Latin America?
Ethical considerations include ensuring that participants fully understand treatment options before agreeing to participate, as there is a risk of patients consenting without thorough discussions.
What percentage of the worldwide research market does Latin America represent?
As of 2023, Latin America represents 2.1% of the worldwide research market, with expectations for growth as global organizations recognize the region's potential.
What are the cost advantages of conducting trials in Latin America?
There are significant labor and facility cost savings compared to the United States, with an average of 83% of studies in the region being industry-sponsored, making it appealing for pharmaceutical companies seeking cost-effective solutions.
How does the prevalence of targeted diseases in Latin America affect clinical trials?
The high prevalence of targeted diseases improves patient accessibility and facilitates quicker recruitment and retention of participants, which is crucial for the success of clinical trials.
What is Colombia's plan for fostering research and innovation in the region?
Colombia is actively pursuing an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy, demonstrating its commitment to creating an environment conducive to research.




