Overview
Medtech companies should prioritize early feasibility studies (EFS) as they provide critical insights into product functionality, safety, and user acceptance, ultimately enhancing the likelihood of successful product launches. The article supports this by detailing how EFS facilitates iterative testing, risk mitigation, and regulatory compliance, thereby fostering innovation and increasing stakeholder confidence in new medical technologies.
Introduction
In the rapidly evolving landscape of medical technology, early feasibility studies (EFS) have emerged as a critical component for assessing the viability of innovative products before significant investments are made. These studies provide invaluable insights into product functionality, safety, and user acceptance, enabling companies to refine their offerings based on real-world feedback.
For instance, ReGelTec, Inc.'s recent EFS for HYDRAFIL™ in Colombia not only showcased successful patient treatments but also highlighted the innovative use of remote proctoring for clinical trials.
As the medtech sector grapples with regulatory complexities and market demands, prioritizing early feasibility assessments becomes essential for mitigating risks, enhancing stakeholder confidence, and ensuring regulatory compliance.
This article delves into the strategic importance of EFS, exploring how they serve as a foundation for informed decision-making, foster innovation, and ultimately pave the way for successful product launches in a competitive market.
The Strategic Importance of Early Feasibility Studies in Medtech
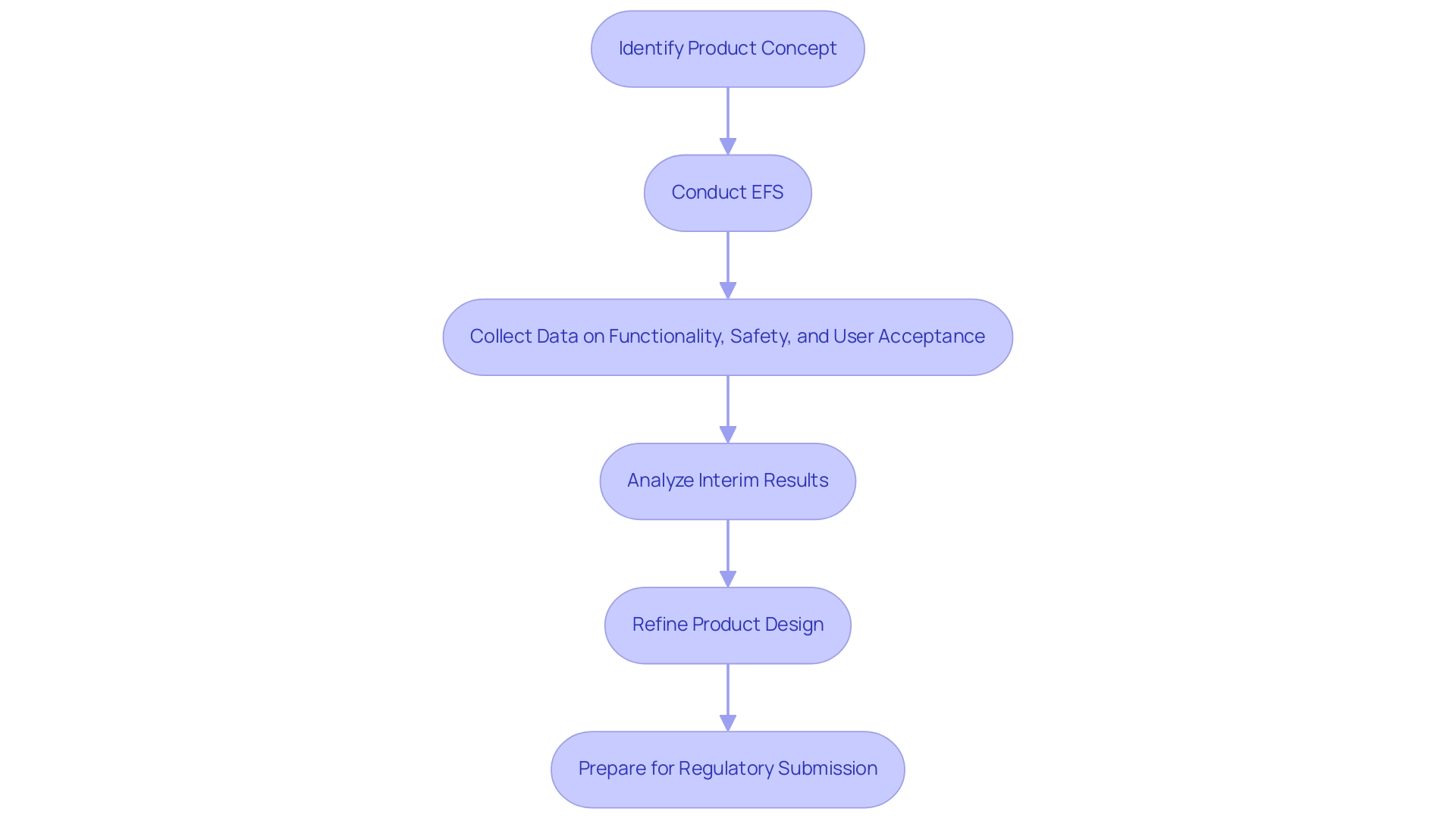
In the medtech sector, understanding why medtech companies should prioritize early feasibility studies (EFS) is essential for evaluating the viability of innovative products before making significant financial commitments. These studies enable companies, such as ReGelTec, Inc., to collect vital information regarding product functionality, safety, and user acceptance, as demonstrated in their recent EFS for HYDRAFIL™ in Colombia, where eleven patients with degenerative disc disease were successfully treated. The procedures were proctored remotely via Zoom, showcasing innovative practices in clinical trial management.
By obtaining this data early in the development process, medtech firms can make informed decisions that significantly shape product design and development. For instance, the EFS process, established by the FDA, facilitates the assessment of novel medical devices in a controlled clinical environment, typically involving fewer than 10 patients. This regulatory pathway is designed to evaluate device performance and refine user techniques, allowing for iterative design improvements based on real-world feedback.
Success in these investigations hinges on the careful selection of researchers and adaptable protocols, which can be rapidly adjusted based on interim results. As noted by Clinical Program Scientist Mike Otlewski, 'For more information about MED Institute and how we can partner with you, please visit medinstitute.com or contact us directly at askmed@medinstitute.com | 855.463.1633.' Understanding why Medtech companies should prioritize early feasibility studies is essential, as these assessments help in identifying and mitigating risks while also bolstering stakeholder confidence.
These studies exemplify a commitment to rigorous research and compliance, ultimately paving the way for a more streamlined approval process. Furthermore, the 21st Century Cures Act, enacted in 2016, has significantly influenced regulatory dynamics within the medtech sector. Recent updates regarding the EFS Program highlight opportunities for interactive review and support in submission preparation, further improving the practicality of bringing innovative products to market.
The focus on initial assessments is essential for enhancing medtech product success rates, highlighting why medtech companies should prioritize early feasibility studies, as they offer vital insights that can foster the development of only the most promising concepts in the medical technology field.
Key Benefits of Prioritizing Early Feasibility Studies for Medtech Companies
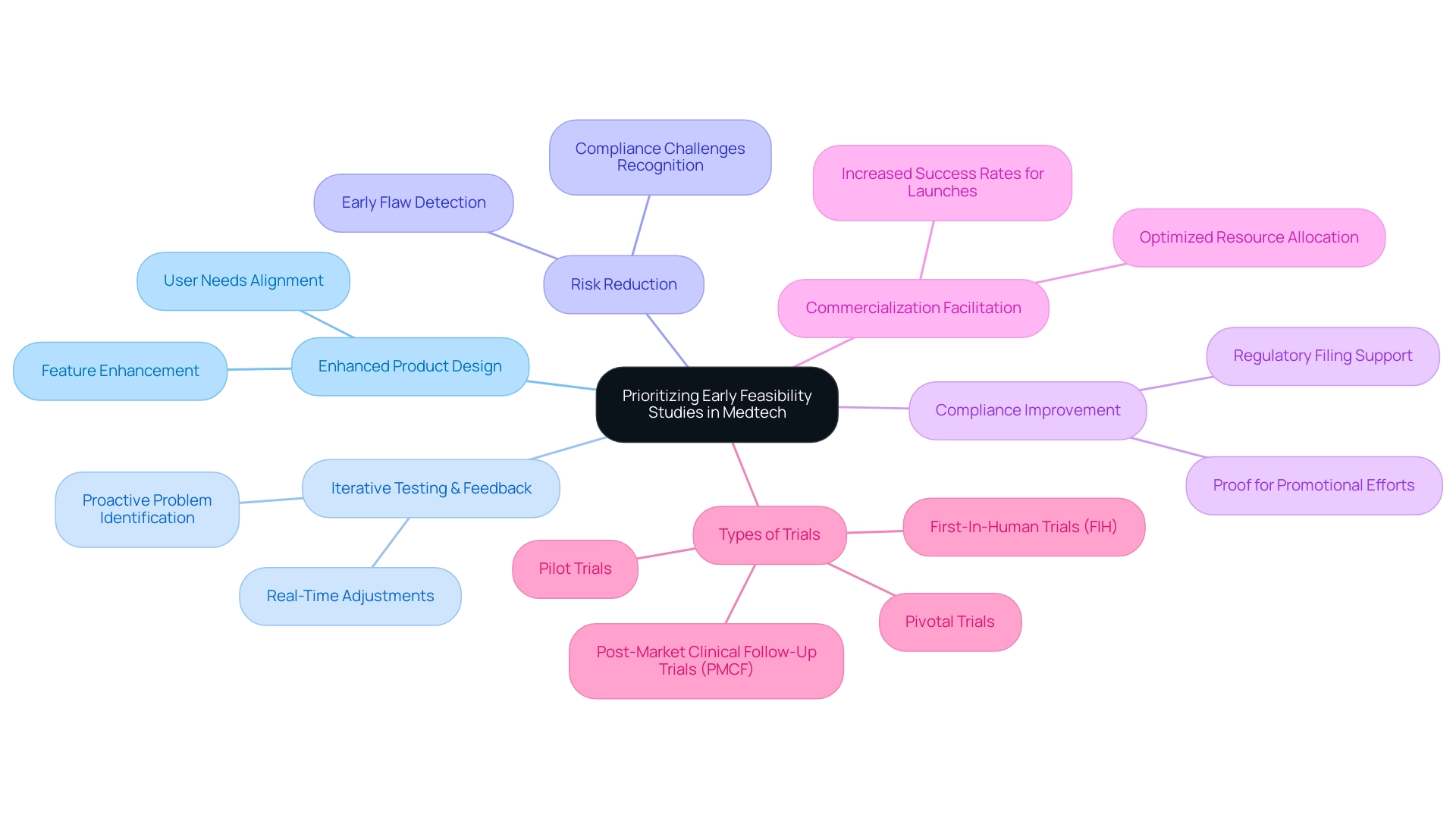
The discussion on why medtech companies should prioritize early feasibility studies emphasizes numerous benefits for medtech firms, particularly in enhancing product design and market readiness. These studies highlight why medtech companies should prioritize early feasibility studies, as they enable iterative testing and real-time feedback, fostering innovations that align closely with user needs. This flexible method not only results in enhanced product features but also reduces the risks linked to product development by recognizing possible flaws or compliance challenges at a preliminary stage.
By tackling these challenges proactively, companies can make timely adjustments that improve compliance and product efficacy. Furthermore, initial assessments offer essential proof that aids both promotional efforts and regulatory filings, facilitating the commercialization process. This strategic focus on why medtech companies should prioritize early feasibility studies not only optimizes resource allocation but also significantly boosts the likelihood of successful product launches.
As emphasized by bioaccess®, a frontrunner in Medtech clinical research with over 20 years of expertise, the knowledge acquired from trials can assist in modifying successful interventions for new populations or environments, offering valuable perspectives on their effectiveness. Additionally, bioaccess® manages a range of research efforts including:
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Trials (PMCF)
ensuring comprehensive support throughout the clinical trial process. It is also significant to acknowledge the constraints in the current database regarding initial assessments, especially because of the absence of required registration for small clinical trials, which can influence the overall comprehension of these assessments' implications in the medtech landscape.
As Dr. Valentin Fuster, Editor-in-Chief, highlights, understanding the extensive framework of early assessments is essential for fostering innovation and attaining a competitive edge in the medtech industry, which emphasizes why medtech companies should prioritize early feasibility studies.
Regulatory Compliance and Risk Management through Early Feasibility Studies
Initial assessments are crucial for guaranteeing adherence to regulations and efficient risk management in the medtech field, highlighting why medtech companies should prioritize early feasibility studies. Recognizing compliance demands from the beginning of product creation highlights why medtech companies should prioritize early feasibility studies to align their innovations with established standards. This proactive strategy not only mitigates risks associated with non-compliance—which can lead to costly delays, fines, or product recalls—but also illustrates why medtech companies should prioritize early feasibility studies to enhance the overall integrity of the development process.
Understanding why medtech companies should prioritize early feasibility studies enables valuable discussions with oversight authorities, offering essential guidance and insights that simplify approval processes and enhance research quality.
In Latin America, bioaccess® provides extensive clinical trial management services, including:
- Site viability evaluations
- Investigator selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting on trial status, inventory, and adverse events
Their expertise encompasses research areas, including why medtech companies should prioritize early feasibility studies, first-in-human, pilot, pivotal, and post-market follow-up, effectively tackling the intricate challenges encountered by medical device startups, such as compliance obstacles, competition, recruitment issues, and financial limitations.
Additionally, as emphasized by the case analysis on cybersecurity, incorporating connected medical devices into healthcare IT systems requires a proactive strategy to cybersecurity, involving risk evaluations and ongoing monitoring. Bioaccess®'s proficiency in overseeing clinical trials can assist in reducing these cybersecurity threats, guaranteeing that all compliance demands are fulfilled and that research is carried out safely. This highlights the necessity for ongoing employee training and education on current compliance requirements and best practices.
Furthermore, the changing post-market oversight trends, especially concerning market monitoring and data privacy, highlight why medtech companies should prioritize early feasibility studies in the dynamic environment in which initial assessments operate. As Dr. Wayne A. Taylor states, "This book is designed for engineers and scientists in the medical device industry," emphasizing the importance of a thorough understanding and adherence to compliance frameworks for successful outcomes. In 2024, the importance of initial assessments in risk management approaches remains crucial, highlighting why medtech companies should prioritize early feasibility studies as compliance challenges continue to develop.
Enhancing Stakeholder Engagement and Investor Confidence
Engaging stakeholders and fostering investor confidence are critical elements in the successful development of medical technologies. Initial assessments, a key focus of bioaccess®, play a pivotal role by providing concrete data and insights that can be effectively communicated to stakeholders, illustrating the viability and potential success of a product. This level of transparency not only cultivates trust but also promotes collaboration among researchers, investors, and regulatory agencies.
Additionally, our thorough clinical trial management services include:
- Viability assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that all elements of the research are handled effectively. Notably, there is a lack of guidance for managing conflicts of interest in multi-stakeholder engagement, which is crucial for maintaining integrity and trust in these processes. A recent trend observed in participatory research indicates a concentration of investigations in Europe and North America, suggesting a gap in global representation that medtech companies, particularly in Latin America, can address by enhancing their stakeholder engagement strategies.
For example, a global medtech client has deployed ethnographic research to gain insights into stakeholders and has established a harmonized customer journey taxonomy to prioritize actions on key pain points. Furthermore, as Victoria Bough notes,
Across industries, CX leaders have seen 15 to 20 percent increases in sales conversion rates, 20 to 50 percent declines in service costs, and 10 to 20 percent improvements in customer satisfaction.
There are compelling reasons for why medtech companies should prioritize early feasibility studies, as it allows them to create a narrative around their innovations that is essential for attracting funding and support in an increasingly competitive market.
Establishing investor confidence through these analyses not only helps in obtaining financial support but also aligns with strategic objectives for advancing medical technologies and enhancing local economies through job creation and healthcare improvement. With over 20 years of expertise in managing medical device clinical trials, bioaccess® specializes in:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This highlights why medtech companies should prioritize early feasibility studies to navigate regulatory environments effectively.
Facilitating Innovation and Market Adaptability
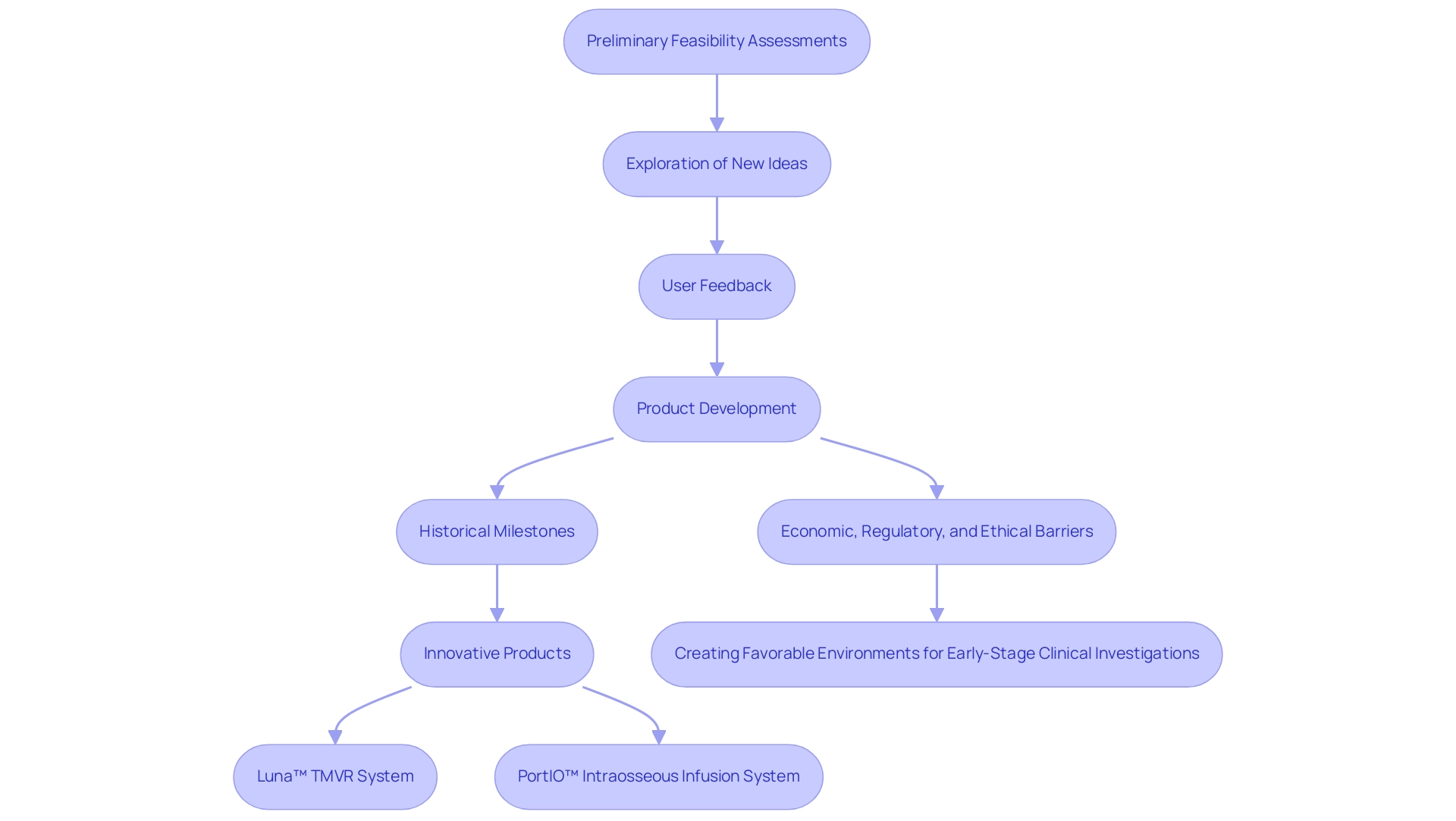
Preliminary feasibility assessments are instrumental in driving innovation and enhancing market adaptability, highlighting why Medtech companies should prioritize early feasibility studies. By offering a platform for exploring new ideas and technologies without the limitations of substantial upfront investment, this research fosters a culture of experimentation and iterative design. Feedback from potential users becomes a crucial element in informing product development, enabling companies to fine-tune their offerings in response to real-world applications.
Historical milestones, such as the first human TAVR procedure conducted on April 16, 2002, highlight the significance of innovation in Medtech, demonstrating how initial research can result in groundbreaking advancements like Tioga Cardiovascular's Luna™ TMVR System, which has recently entered first human trials, and PAVmed's successful implementation of the PortIO™ Intraosseous Infusion System in Colombia. As market demands evolve, organizations that emphasize initial assessments are uniquely positioned to pivot and adapt their products, ensuring they stay pertinent and competitive. This proactive approach to innovation not only addresses current healthcare needs but also anticipates future trends, thereby solidifying their standing within the industry.
The role of bioaccess® as a specialist Medtech CRO is vital, providing economical solutions for clinical trials in Latin America, including approval processes, patient recruitment, and prompt data delivery. For instance, bioaccess® provides tailored strategies that reduce trial costs while maintaining high-quality standards. Helen Banks aptly noted, 'We wish to thank Helen Banks for providing language assistance,' highlighting the collaborative efforts in advancing Medtech discussions.
Furthermore, the promising trajectory of medical device innovation, while constrained by economic, regulatory, and ethical barriers, illustrates why Medtech companies should prioritize early feasibility studies, as emphasized by the case study titled 'Expert Opinions on EFS Implementation,' which highlights the importance of creating a favorable environment for early-stage clinical investigations. This paves the way for more effective and timely solutions.
Conclusion
Early feasibility studies (EFS) serve as a cornerstone for success in the medtech industry, offering critical insights that inform product design, enhance regulatory compliance, and foster innovation. By gathering real-world data early in the development process, companies can refine their offerings and address potential challenges proactively, thereby mitigating risks and improving stakeholder confidence. The case of ReGelTec, Inc.'s HYDRAFIL™ highlights the tangible benefits of EFS, illustrating how innovative practices, such as remote proctoring, can enhance clinical trial management and patient outcomes.
Moreover, prioritizing EFS not only streamlines the commercialization process but also positions companies to adapt to market demands and regulatory changes effectively. The strategic focus on these studies strengthens the overall integrity of the development process, ensuring that innovations align with user needs and regulatory standards. As the medtech landscape continues to evolve, the emphasis on early feasibility assessments will remain vital for driving innovation, securing investor confidence, and achieving successful product launches.
In conclusion, the integration of early feasibility studies into the medtech development cycle is not merely advantageous; it is essential for navigating the complexities of the industry. By leveraging the insights gained from EFS, medtech companies can enhance their competitiveness, foster collaboration among stakeholders, and ultimately contribute to advancements in healthcare that benefit society as a whole. The commitment to rigorous research and iterative design processes established through EFS will lay the groundwork for the next generation of medical technologies.
Frequently Asked Questions
What are early feasibility studies (EFS) in the medtech sector?
Early feasibility studies (EFS) are assessments conducted to evaluate the viability of innovative medical products before significant financial commitments are made. They help gather crucial information on product functionality, safety, and user acceptance.
Why should medtech companies prioritize early feasibility studies?
Medtech companies should prioritize EFS because they provide essential insights that shape product design and development, identify and mitigate risks, and enhance stakeholder confidence, ultimately improving the chances of successful product launches.
How do early feasibility studies impact product design?
EFS facilitate iterative testing and real-time feedback, allowing companies to make timely adjustments to improve compliance and product efficacy, leading to enhanced product features that align closely with user needs.
What is the role of the FDA in early feasibility studies?
The FDA established the EFS process to assess novel medical devices in controlled clinical environments, typically involving fewer than 10 patients. This regulatory pathway helps evaluate device performance and refine user techniques based on real-world feedback.
How does the 21st Century Cures Act influence early feasibility studies?
The 21st Century Cures Act has influenced regulatory dynamics by highlighting opportunities for interactive review and support in submission preparation, improving the practicality of bringing innovative products to market.
What types of trials does bioaccess® manage in the medtech clinical research process?
Bioaccess® manages various research efforts, including First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF), providing comprehensive support throughout the clinical trial process.
What are the benefits of conducting early feasibility studies?
The benefits include enhanced product design, reduced development risks, improved compliance, essential proof for promotional efforts and regulatory filings, and optimized resource allocation, which collectively boost the likelihood of successful product launches.
What are the constraints regarding initial assessments in the current medtech landscape?
One constraint is the absence of required registration for small clinical trials, which can influence the overall understanding of the implications of these assessments within the medtech sector.




