Overview
Best practices for clinical research site management focus on establishing clear objectives, defining roles, and employing effective project management strategies to ensure compliance and enhance operational efficiency. The article supports this by detailing essential activities such as patient recruitment, data collection, and the use of technology, emphasizing that structured management and ongoing training are crucial for adapting to the evolving landscape of clinical research.
Introduction
In the intricate world of clinical research, effective site management is paramount to the success of trials that can ultimately change lives. From navigating complex regulatory landscapes to implementing robust recruitment strategies, clinical research teams face a myriad of challenges that require meticulous planning and execution.
As the demand for innovative therapies grows, so does the need for efficient management practices that ensure compliance, enhance patient engagement, and leverage cutting-edge technology.
This article delves into the fundamentals of clinical research site management, exploring key strategies and trends that empower teams to optimize their operations and achieve successful outcomes in an ever-evolving field.
Fundamentals of Clinical Research Site Management
Clinical research site management is a multifaceted process that includes essential activities such as project initiation, patient recruitment, data collection, and compliance monitoring. Effective management starts with a thorough comprehension of research protocols and regulatory standards, particularly understanding confounding variables, as highlighted by the 10% rule. This rule states that a variable is a confounder if the regression coefficient for the exposure variable changes by more than 10% with the inclusion of the possible confounder in the model.
Essential principles guiding effective clinical research site management include:
- Establishing clear objectives
- Defining individual roles and responsibilities
- Creating a structured timeline
Our comprehensive services in clinical research site management include:
- Feasibility assessments
- Site selection
- Review and feedback on documents to comply with country requirements
- Trial setup
- Import permits
- Project management
- Ongoing reporting on trial status
- Inventory
- Serious and non-serious adverse events
These services ensure that trials comply with local regulations and standards. As A.M. noted, Y.P., P.Y., and A.M. made significant contributions to the research design, analysis, and interpretation of the data, critically reviewed the manuscript, and approved the final manuscript as submitted, underscoring the importance of collaboration and accountability among team members.
Moreover, ongoing training and updates regarding best practices are vital for teams to remain aligned with the rapidly changing research landscape. Recent trends indicate a notable shift towards advanced monitoring methods, as evidenced in a comparative study titled 'Comparison of Monitoring Methods,' which highlighted a growing preference for centralized and remote monitoring approaches. These techniques not only boost efficiency and data integrity but also optimize performance and enhance patient recruitment efforts, ensuring a more effective research process.
Furthermore, Colombia's competitive strengths—such as cost effectiveness, regulatory agility, high-quality healthcare, and R&D tax incentives—further enhance the potential for successful first-in-human studies.
BOOK A MEETING
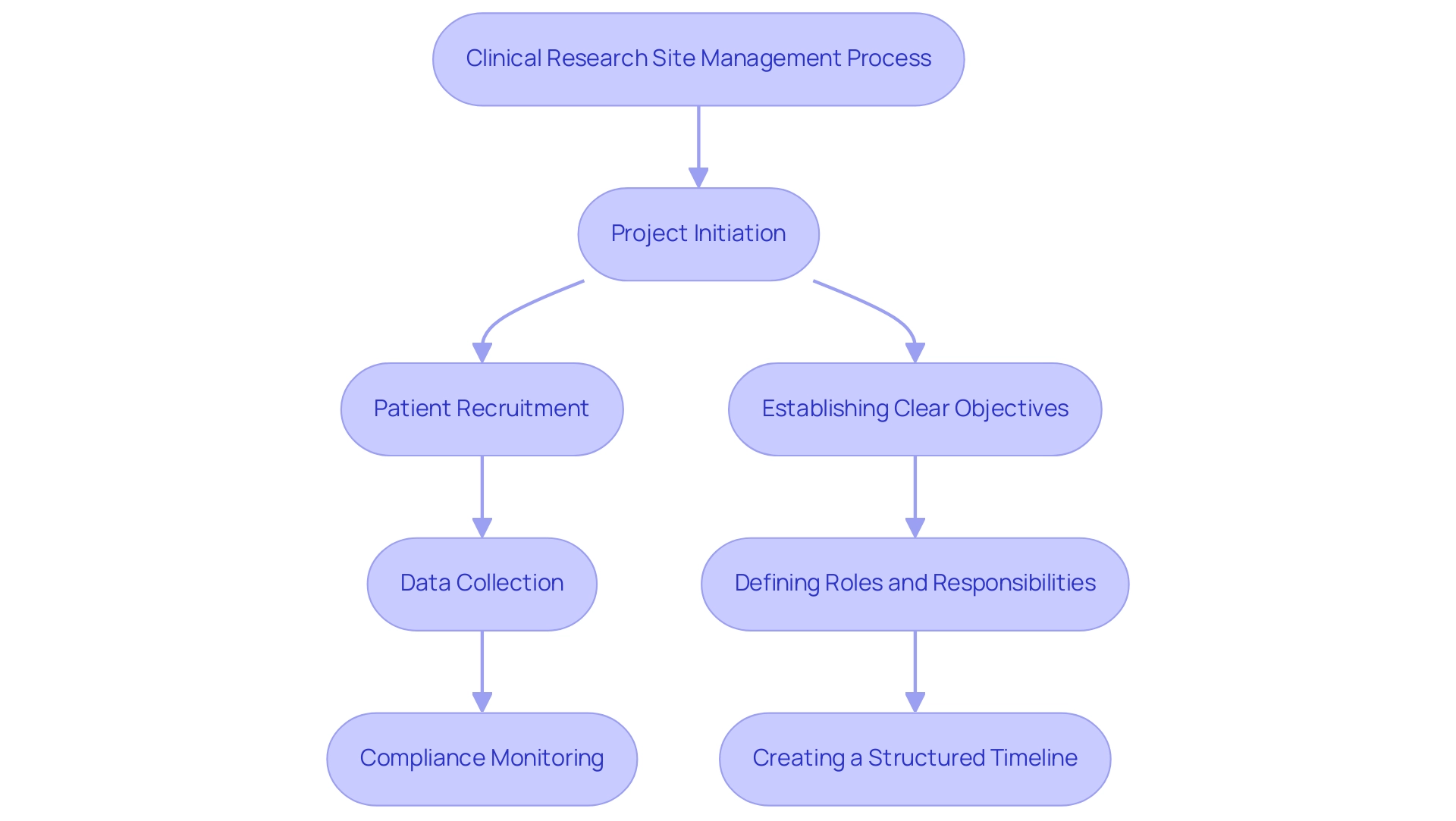
Ensuring Compliance: Navigating Regulations and Protocols
Ensuring compliance within clinical research site management necessitates a thorough understanding of applicable regulations and the establishment of robust standard operating procedures (SOPs). The terrain of medical research is changing, with nearly 200 studies of psychedelics currently listed, highlighting the potential and intricacy of contemporary studies. To effectively reach diverse patient populations, sponsors should leverage technology, such as digital recruitment platforms and telehealth solutions, which enhance patient engagement and streamline the enrollment process.
Our extensive clinical research site management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
This ensures strong oversight and adherence to regulatory requirements. Project management is critical for coordinating all aspects of the trial, while thorough reporting on study status, inventory, and adverse events is essential for transparency and accountability. Regular audits and comprehensive training sessions are critical in reinforcing a compliance-oriented culture within study teams.
Open communication with regulatory bodies, such as INVIMA—the Level 4 health authority acknowledged by PAHO/WHO—is essential for proactively addressing concerns, thereby fostering a transparent environment. Implementing a compliance checklist for each project can effectively track adherence to protocols and identify areas for improvement. A pertinent case study titled 'Psychedelics in Clinical Trials' illustrates the emerging field, revealing opportunities to address unmet medical needs and fostering optimism for advancements in treating central nervous system disorders.
By prioritizing compliance, study sites not only build trust with stakeholders but also enhance the credibility of their findings. As mentioned by Ken Getz, executive director and professor at Tufts University School of Medicine, it is essential for professionals in the field to continually examine compliance statistics and tackle factors affecting eligibility in research studies. This examination is essential for guaranteeing that varied groups are sufficiently represented in medical studies.
Moreover, groups such as the Task force, co-directed by Mr. Keyes, promote high standards of transparency and compliance, emphasizing the shared duty of the academic community to maintain these principles. Furthermore, the significance of import permits and the nationalization of investigational devices cannot be exaggerated, as they are essential to ensuring that evaluations adhere to local regulations and promote the seamless functioning of research.
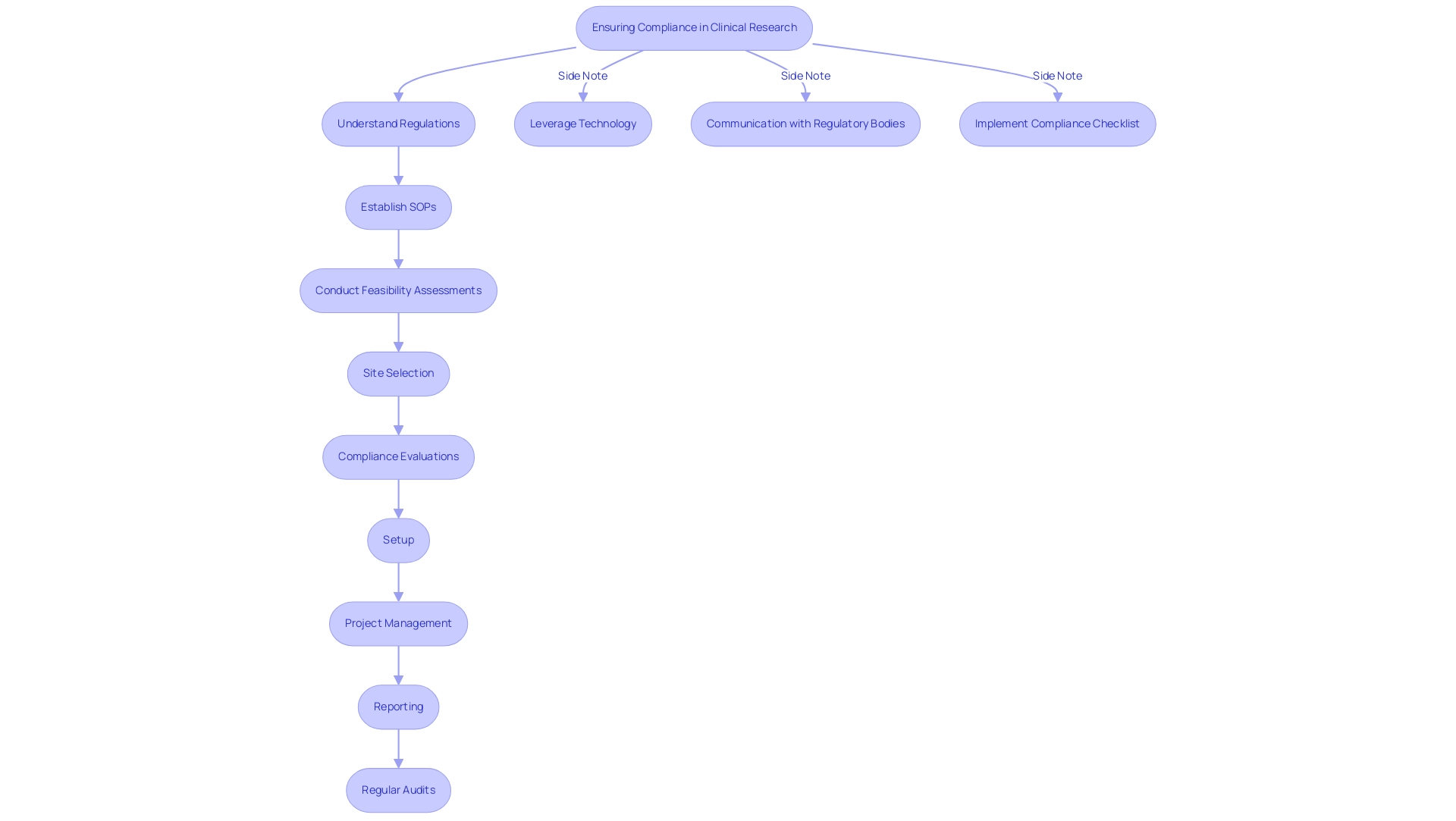
Effective Recruitment Strategies for Clinical Research Teams
To successfully recruit qualified researchers, research sites must adopt a multi-faceted strategy that effectively resonates with potential candidates. Engaging in networking opportunities at industry conferences, leveraging job boards, and forming collaborations with academic institutions are essential tactics for identifying promising talent. Furthermore, our extensive services in clinical research site management—including feasibility evaluations, site selection, compliance assessments, setup, import permits, project management, and reporting—are vital in aiding these recruitment efforts.
For instance, the setup process ensures that all logistical aspects are handled efficiently, while compliance reviews guarantee adherence to regulatory requirements, thereby attracting candidates who value a well-structured environment. Crafting a compelling job description that emphasizes the site’s mission and core values is vital for attracting individuals who share the organization’s vision. In 2024, statistics indicate that 30% of registered recruiting studies worldwide are located in both U.S. and non-U.S. regions, underscoring the necessity for a diverse recruitment approach that addresses the unique challenges of each area.
Offering competitive compensation packages alongside robust professional development opportunities significantly enhances recruitment efforts, contributing to the economic growth and healthcare improvement in local communities. Furthermore, with the rise of decentralized studies, maintaining patient engagement remains a challenge, necessitating innovative strategies to ensure participants feel connected to the study. Once researchers are hired, it is vital to provide comprehensive onboarding and mentorship.
As noted by a Principal Investigator, 'Mentorship and supervision were integral to the program, with the Principal Investigator providing ongoing guidance and support throughout the clinical trial.' Such support not only helps new team members feel connected but also fosters a productive and engaged atmosphere from the outset. Furthermore, findings from the case examination titled 'Barriers to Recruitment in New Healthcare Schools' indicate that new institutions encounter distinct challenges, including restricted experience among faculty and students.
By implementing effective hiring approaches from this analysis, these institutions can improve their recruitment initiatives and create more robust teams, positively influencing the local economy and promoting global collaboration.
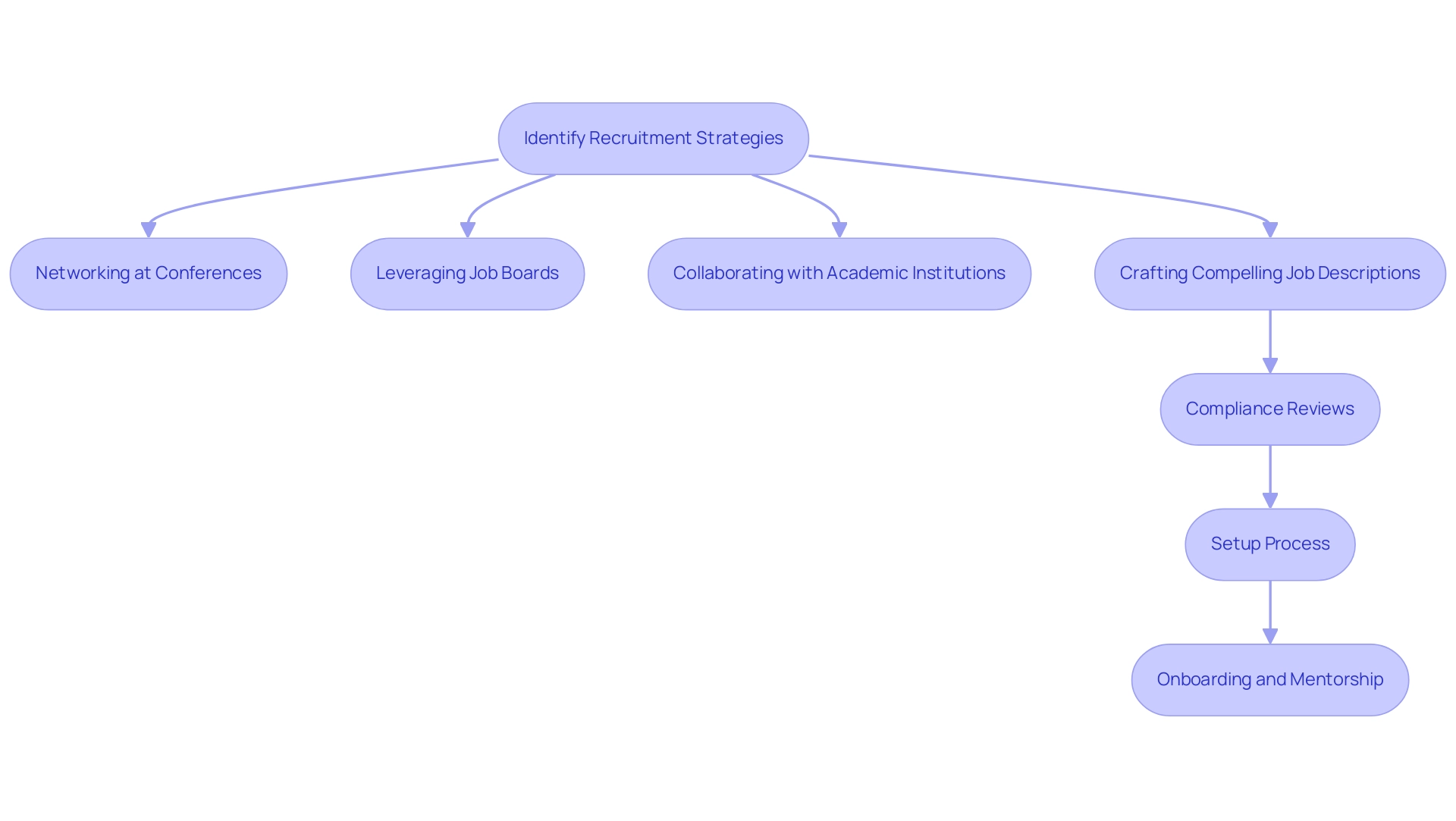
Balancing Multiple Projects: Time Management Techniques
Successfully managing several medical studies relies on the principles of clinical research site management, which requires setting clear priorities and schedules. The integration of comprehensive project management tools is vital for effective clinical research site management, as they enable teams to monitor progress and allocate resources efficiently across various services, including:
- Feasibility studies
- Site selection
- Trial setup
Regular team meetings should be held to evaluate project status and tackle any challenges, fostering accountability and transparency within the group.
Furthermore, adopting a flexible scheduling system empowers team members to adjust to evolving demands without compromising the quality of their work. As Dr. Inger Mewburn notes,
Instead, working in focused blocks of one to two hours with a break after each session helps improve productivity.
This method highlights the importance of time management strategies in health studies.
It is important to note that the link between time management and outcomes is highly heterogeneous, suggesting that further research is necessary to understand the moderating factors at play. Furthermore, tracking essential indicators like enrollment rates and data quality is vital for clinical research site management to meet deadlines in clinical trials, especially in the realm of accelerated medical device research in Latin America. Acknowledging and rewarding team contributions, as emphasized in the case analysis titled 'Motivating Teams in Clinical Trials,' plays a significant role in fostering a positive work environment.
This recognition leads to team members feeling valued and engaged, which directly impacts performance and project outcomes. Furthermore, compliance reviews are essential to ensure that all study documents meet regulatory requirements, and regular reporting on study status and adverse events is critical for maintaining transparency with stakeholders. Lastly, as the field of medical inquiry evolves, challenges such as predatory publishers and plagiarism remain significant issues that require attention.
Ultimately, cultivating a proactive approach to time management not only enhances efficiency but also contributes to successful outcomes across all projects, particularly in the context of bioaccess®'s expertise in clinical research site management, which includes:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
Leveraging Technology for Enhanced Site Operations
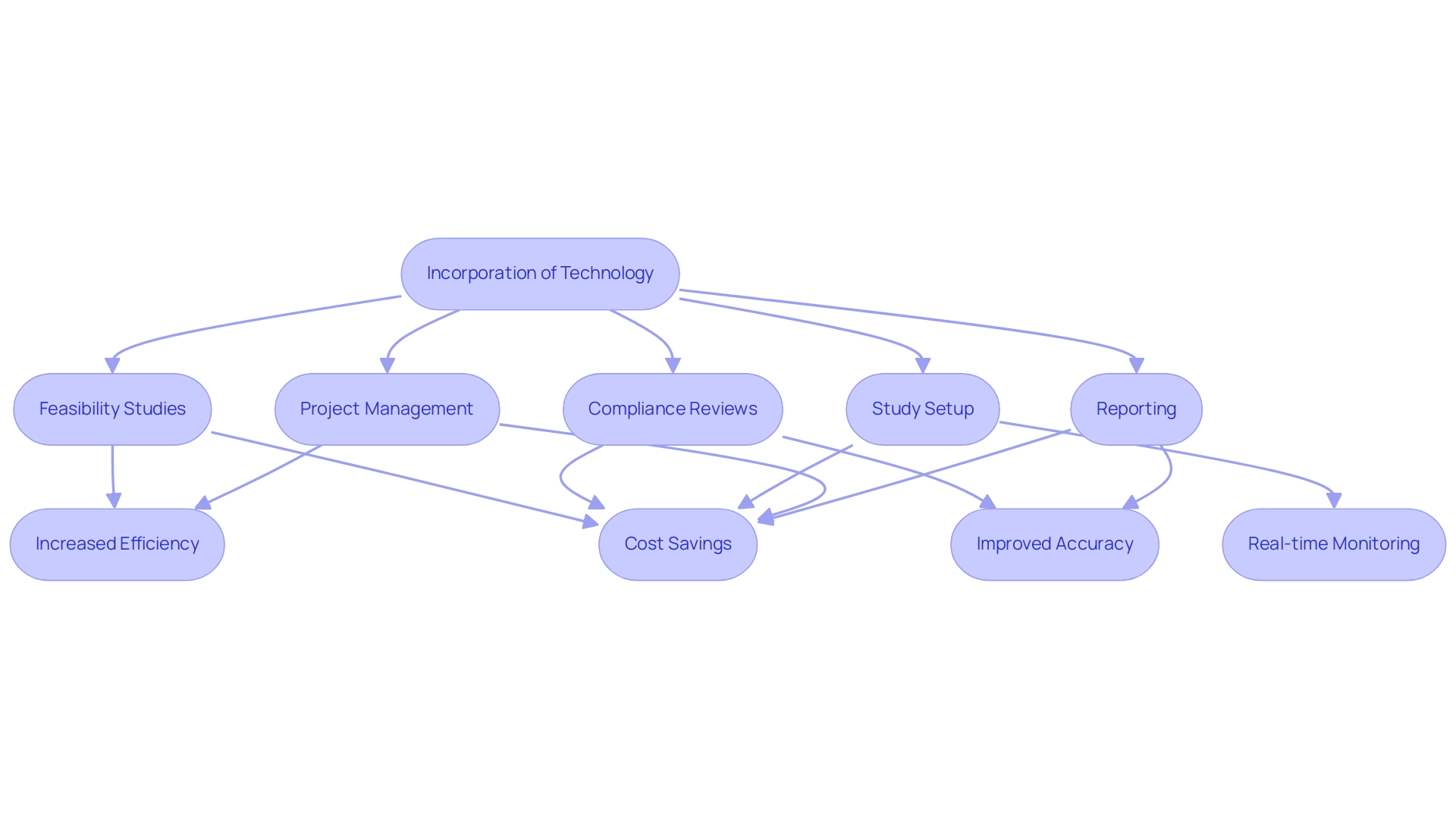
The incorporation of technological solutions like electronic data capture (EDC) systems and research management systems (CTMS) is revolutionizing clinical research site management operations. These tools not only streamline data collection but also enhance accuracy and enable real-time monitoring of progress. Our extensive clinical research site management services include:
- Feasibility studies and site selection
- Compliance reviews
- Study setup involving ethics committee and health ministry approvals
- Import permits
- Project management
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events
As the Dysautonomia market is projected to reach approximately USD 7.8 billion by 2032, leveraging these technologies becomes paramount in meeting increasing demands. According to a recent market report published by Persistence Market Research, revenue from the global communication and collaboration market was US$ 138.5 million in 2012 and US$ 214.8 million in 2016, representing a CAGR of 11.6% from 2012 to 2016. Additionally, recent surveys show that:
- 94% of patients are likely to utilize mobile applications for studies
- 45% find 'bring your own device' (BYOD) options more convenient
This shift underscores the impact of telemedicine in broadening participant access and streamlining patient visits. The adoption of decentralized trials (DCTs) can lead to significant cost savings—estimated between 10-25%—by reducing the need for multiple sites and lowering patient visit expenses, as demonstrated by the case analysis titled 'Time and Cost Savings from DCTs,' which highlights the enhanced patient engagement through BYOD capabilities. To maximize these benefits, training staff on the effective use of EDC and CTMS is essential.
By adopting technology and our extensive service capabilities, including strong reporting systems, clinical research site management can boost operational efficiency, generate employment, aid in economic expansion, and ultimately enhance trial outcomes, as evidenced by successful case studies highlighting technology integration within experimental environments.
Fostering Communication and Teamwork in Research Settings
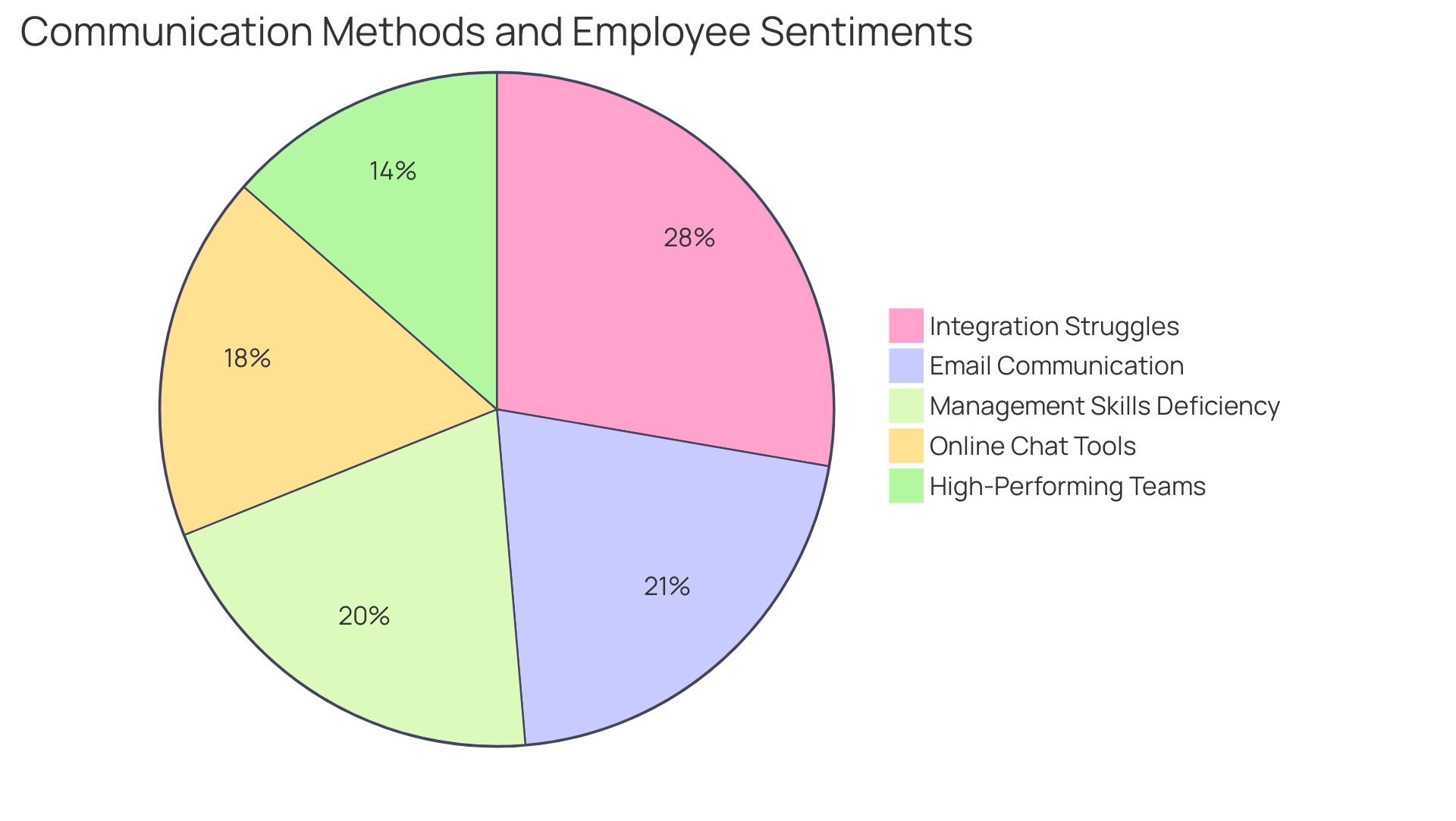
To cultivate effective communication and teamwork within clinical study teams, it is vital to establish regular check-ins and maintain open channels for feedback. Current data reveals that:
- 31% of internal communication occurs via email
- 26% is facilitated through online chat tools
This underscores the importance of utilizing diverse communication platforms. Collaborative tools, such as shared documents and project management platforms, can significantly enhance transparency and engagement among team members.
Furthermore, the 2022 State of Remote Work Report from Owl Labs highlights that:
- 41% of remote employees struggle to feel integrated into their company’s culture
This emphasizes the need for strategies that foster connection, such as team-building activities. Recent findings indicate that nearly:
- 30% of employees feel that their manager lacks essential team-building skills, as noted in the 2019 People Management Report
This suggests that organizations should invest in training for managers to develop these capabilities, which is crucial since only:
- 20% of executives in a McKinsey study believed their team was high-performing
Recognizing individual contributions can also strengthen relationships and boost morale. Additionally, utilizing tools like AIScreen, which offers detailed analytics to measure the effectiveness of communication strategies, can provide valuable insights. By fostering a culture of collaboration and effective communication, sites can enhance problem-solving abilities and, ultimately, drive greater success in their studies.
Continuous Improvement: Learning from Experience and Feedback
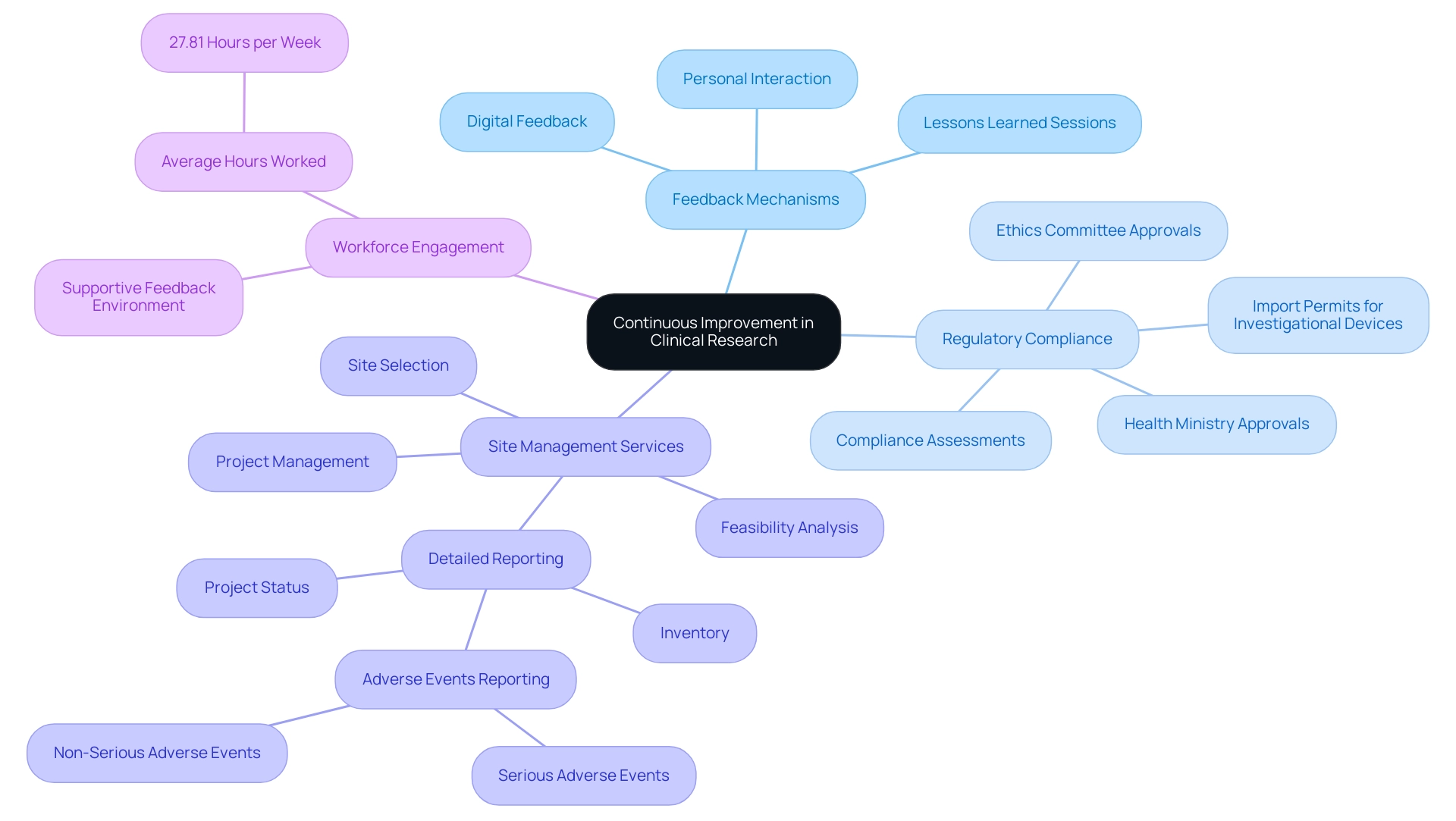
To enhance clinical research outcomes, effective clinical research site management must establish strong systems for gathering and analyzing feedback from team members, participants, and stakeholders, alongside a comprehensive suite of clinical management services. Our capabilities encompass:
- Feasibility analysis
- Site selection
- Compliance assessments to ensure adherence to regulatory standards
- Setup processes that involve:
- Ethics committee approvals
- Health ministry approvals
- Import permits for investigational devices
- Project management
- Detailed reporting on:
- Project status
- Inventory
- Both serious and non-serious adverse events
These elements are critical for ensuring that trials not only meet regulatory requirements but also address local needs effectively.
Regular evaluations of learning outcomes, coupled with a thorough identification of areas for improvement, play a pivotal role in informing future practices. For instance, recent findings from a mixed factorial ANOVA indicate that individuals involved in research exhibit significantly higher task engagement when feedback is sourced from a person rather than through digital means, underscoring the value of personal interaction in the feedback process. Significantly, the research revealed that participants showed higher task engagement levels when receiving feedback from an individual, contrasting with the no-feedback condition, which highlights the necessity of personal involvement in the feedback loop.
Furthermore, the average hours worked per week by nurses in healthcare environments is 27.81 hours, highlighting the importance of workforce engagement and the need for effective feedback mechanisms to support their efforts. Incorporating lessons learned sessions encourages open dialogue about the challenges faced during research and cultivates a culture of innovation. As noted by one expert, 'If these things all matter, shouldn’t they be shared in a relatively comparable frequency?'
This sentiment underscores the necessity of consistent communication. By adopting continuous improvement practices in clinical research site management, research sites not only refine their processes but also adapt to the changing environment of research, ultimately contributing to the advancement of medical knowledge and enhancing trial outcomes. Additionally, the impact of Medtech clinical studies extends to local economies, fostering job creation, economic growth, and healthcare improvement through international collaboration and innovation.
Conclusion
Effective site management in clinical research is foundational to achieving successful trial outcomes. By understanding the multifaceted nature of this process—including study initiation, patient recruitment, data collection, and compliance monitoring—research teams can navigate the complexities of regulatory landscapes and enhance patient engagement. The emphasis on clear objectives, defined roles, and structured timelines ensures that all team members are aligned and accountable, which is crucial for maintaining the integrity of clinical trials.
Moreover, leveraging technology and innovative recruitment strategies can significantly improve operational efficiency. The integration of electronic data capture systems and clinical trial management platforms not only streamlines data collection but also fosters real-time monitoring, thereby enhancing the accuracy and reliability of study results. As the landscape of clinical research continues to evolve, embracing these advancements will empower teams to meet the increasing demands for innovative therapies.
Lastly, fostering open communication and a culture of continuous improvement is essential for cultivating effective teamwork and driving research success. By regularly collecting feedback and implementing lessons learned, clinical research sites can adapt their practices to address emerging challenges and improve future outcomes. Overall, prioritizing effective site management practices will not only enhance the quality of clinical trials but also contribute to the advancement of medical science and the betterment of patient care.
Frequently Asked Questions
What is clinical research site management?
Clinical research site management is a multifaceted process that involves activities such as project initiation, patient recruitment, data collection, and compliance monitoring, ensuring that trials adhere to regulatory standards.
What are the key activities involved in clinical research site management?
Key activities include feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and ongoing reporting on trial status and adverse events.
What are the essential principles guiding effective clinical research site management?
The essential principles include establishing clear objectives, defining individual roles and responsibilities, and creating a structured timeline.
How does the 10% rule relate to clinical research?
The 10% rule states that a variable is considered a confounder if the regression coefficient for the exposure variable changes by more than 10% when the possible confounder is included in the model.
Why is ongoing training important in clinical research site management?
Ongoing training is vital to ensure that teams remain aligned with best practices and adapt to the rapidly changing research landscape.
What recent trends are observed in clinical research monitoring methods?
There is a notable shift towards advanced monitoring methods, with a growing preference for centralized and remote monitoring approaches, which improve efficiency, data integrity, and patient recruitment efforts.
What advantages does Colombia offer for clinical research?
Colombia offers competitive strengths such as cost effectiveness, regulatory agility, high-quality healthcare, and R&D tax incentives, enhancing the potential for successful first-in-human studies.
How can technology enhance patient recruitment in clinical research?
Technology, such as digital recruitment platforms and telehealth solutions, can enhance patient engagement and streamline the enrollment process, allowing sponsors to effectively reach diverse patient populations.
What role does compliance play in clinical research site management?
Compliance ensures strong oversight and adherence to regulatory requirements, building trust with stakeholders and enhancing the credibility of research findings.
How can study sites track adherence to protocols?
Implementing a compliance checklist for each project can effectively track adherence to protocols and identify areas for improvement.
What is the significance of import permits in clinical research?
Import permits are essential for ensuring that evaluations adhere to local regulations and facilitate the smooth functioning of research activities.




