Overview
The article delineates the essential steps for designing effective trials for diagnostic devices, underscoring the significance of clear objectives, suitable study populations, and robust data management strategies. It elaborates on best practices such as:
- Adhering to regulatory requirements
- Engaging stakeholders
- Implementing quality control measures
These elements collectively fortify the reliability and applicability of clinical research outcomes within the medical technology sector, establishing a strong foundation for future advancements in the field.
Introduction
In the realm of medical advancements, the design of clinical trials for diagnostic devices emerges as a critical pillar influencing the success of new technologies. Navigating the complexity of regulatory landscapes and the necessity for rigorous methodologies, researchers face a myriad of challenges to ensure their trials yield meaningful results.
From defining precise research questions to selecting appropriate study populations and endpoints, each aspect of trial design plays a pivotal role in shaping outcomes.
This article delves into the fundamental components of clinical trial design, explores common obstacles encountered in the development of diagnostic devices, and highlights best practices that can enhance trial effectiveness, ultimately paving the way for innovative solutions in healthcare.
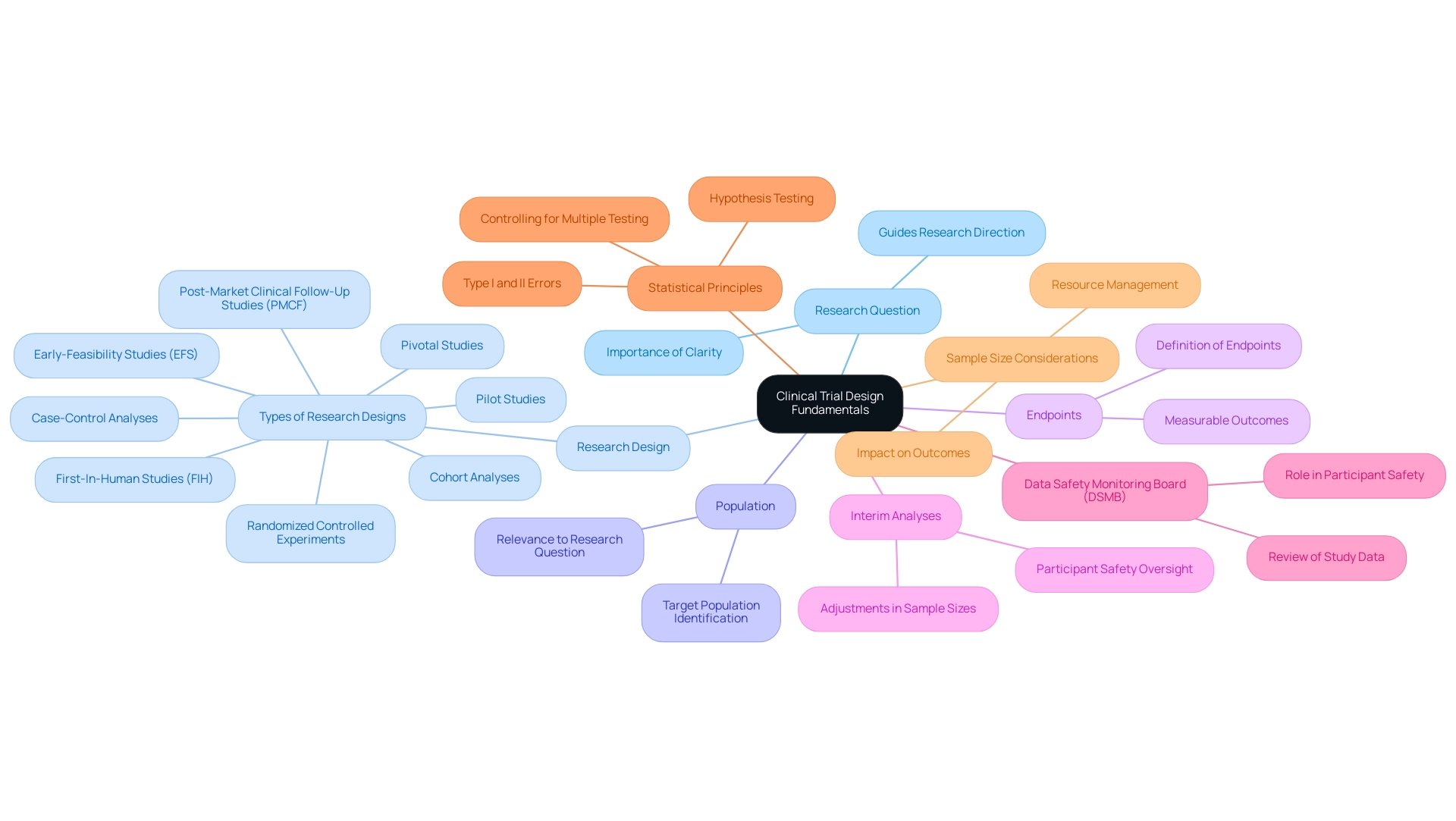
Understanding Clinical Trial Design Fundamentals
Clinical research design represents a systematic approach that delineates the methodology for conducting investigations aimed at addressing specific health-related inquiries. The following key components are essential for developing a robust study framework:
- Research Question: A well-defined research question serves as the cornerstone of any clinical study. It directs the entire research endeavor and establishes the significance of the outcomes measured.
- Research Design: Selecting the appropriate research design is critical. Options include randomized controlled experiments, cohort analyses, and case-control analyses, each catering to distinct research aims and contexts. At bioaccess®, we specialize in a variety of study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring that your research is tailored to meet specific regulatory and scientific needs.
- Population: Identifying the target population is vital. This population must align with the research question to ensure that the findings are applicable and meaningful.
- Endpoints: Clearly defined endpoints are necessary to evaluate the effectiveness of the diagnostic device. These outcomes should be measurable and pertinent to the research question.
Incorporating interim analyses can significantly enhance study design by allowing for adjustments in sample sizes and ensuring participant safety. This process is typically overseen by an independent Data Safety Monitoring Board (DSMB), which plays a crucial role in large or long-duration studies, particularly those with serious safety concerns. The DSMB reviews study data to safeguard participant welfare and assess efficacy.
Recent news highlights that a DSMB should be considered for studies with serious safety concerns, reinforcing the necessity of participant safety oversight.
Recent trends in designing trials for diagnostic devices emphasize the importance of statistical principles, such as hypothesis testing and controlling for multiple testing. For instance, in a placebo-controlled study for multiple sclerosis, assessments from blinded neurologists suggested no benefit of the intervention, underscoring the need for robust study design. A clear understanding of these concepts is essential for researchers to design effective studies and accurately interpret results, ultimately improving the quality of biomedical research.
As emphasized by Scott R. Evans, Ph.D., 'Sample size is a crucial aspect of study design because too large of a sample size wastes resources, while too small of a sample size may lead to inconclusive outcomes.' Thus, a meticulous approach to study design is paramount for designing trials for diagnostic devices and ensuring the successful advancement of medical technology. Moreover, insights from the case analysis titled 'Statistical Concepts in Clinical Research' reveal that grasping fundamental statistical principles, including type I and II errors, is vital for researchers to create strong health investigations and accurately interpret their outcomes.
Furthermore, comprehending the regulatory environment, such as the role of INVIMA as a Level 4 health authority by PAHO/WHO, is crucial for navigating health research in Latin America. With more than 20 years of experience in Medtech, bioaccess® possesses the knowledge and tailored strategy you require to guide your company towards successful research phases, ensuring adherence and efficient project management. Our clients have reported substantial enhancements in experimental efficiency and results, reinforcing our commitment to excellence in the management of studies.
Challenges in Designing Trials for Diagnostic Devices
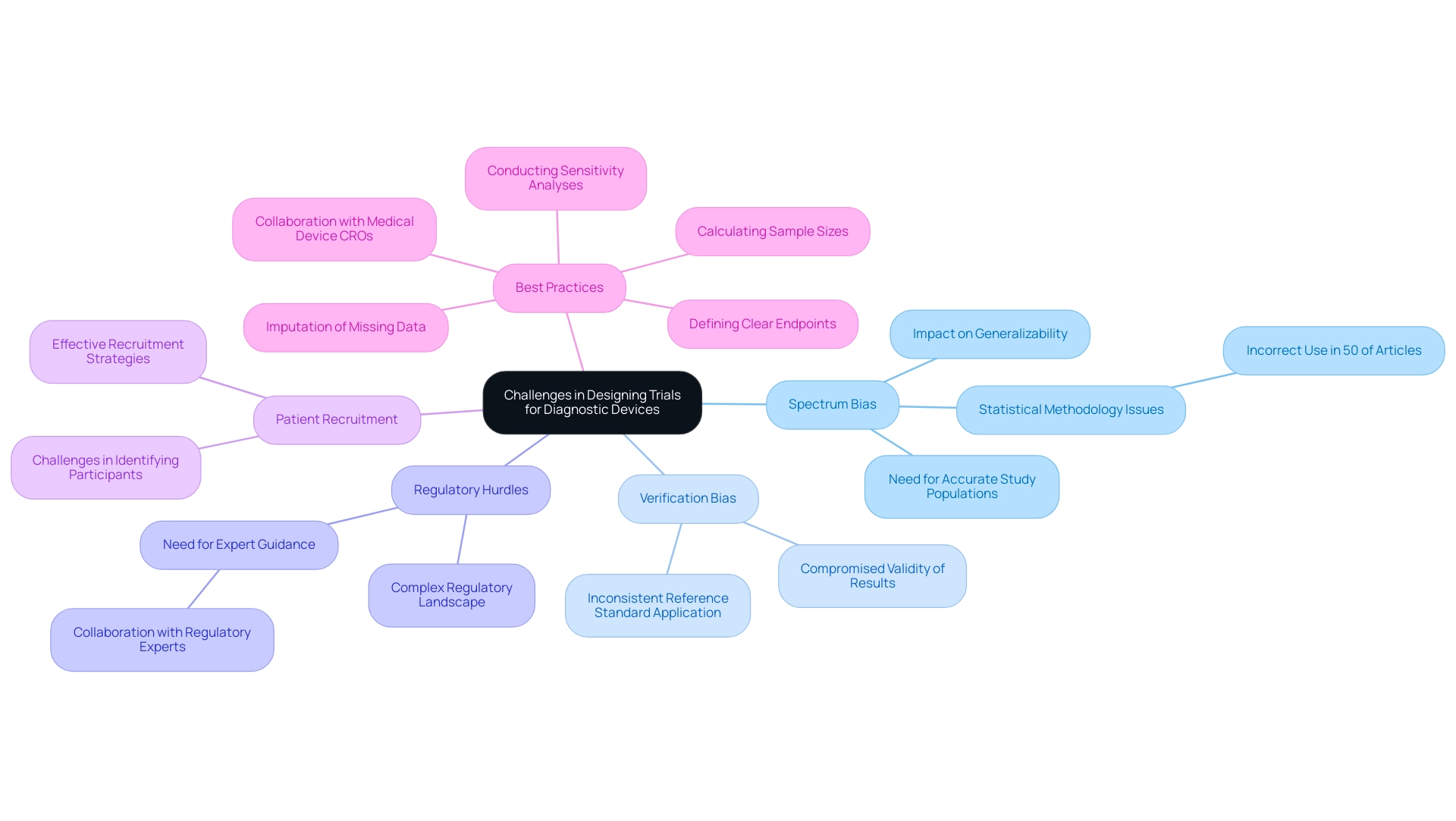
Designing trials for diagnostic devices presents several distinct challenges that can significantly impact the outcomes of clinical research:
- Spectrum Bias: This bias arises when the study population fails to accurately reflect the intended use population, leading to skewed results that may not be generalizable. Approximately half of the published articles in medical journals that employ statistical methods do so incorrectly, exacerbating issues related to spectrum bias. A solid understanding of these issues is crucial for ensuring high-quality clinical studies, as emphasized by Scott R. Evans, Ph.D. from the Department of Statistics at Harvard University.
- Verification Bias: This occurs when the reference standard is not uniformly applied across all participants, compromising the validity of the study results. Ensuring consistent application of the reference standard is essential for maintaining the integrity of the findings.
- Regulatory Hurdles: The complex regulatory landscape for diagnostic instruments presents significant challenges. Navigating these requirements necessitates meticulous planning and adherence to compliance standards, which can be daunting for many research teams. Collaborating with experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can provide invaluable guidance in overcoming these hurdles.
- Patient Recruitment: Identifying suitable participants who meet the inclusion criteria can be particularly challenging, especially in the context of rare conditions. Effective recruitment strategies are essential to ensure a representative sample that can yield reliable results.
Addressing these challenges early in designing trials for diagnostic devices is vital. Implementing best practices, such as defining clear endpoints and calculating precise sample sizes, enhances the likelihood of success. A case analysis titled "Best Practices for Medical Equipment Clinical Evaluations" emphasizes that following these best practices results in more dependable evaluation outcomes and promotes the safe and effective use of medical apparatus.
Additionally, incorporating imputation of missing data and conducting sensitivity analyses are critical aspects of study design that can further strengthen the findings. Partnering with a specialized Medical Device CRO like bioaccess can offer valuable insights and assistance, ultimately resulting in more trustworthy research outcomes and promoting the safe and effective use of diagnostic tools.
The author expresses gratitude to Dr. Justin McArthur and Dr. John Griffin for their invitation to participate in the ANAs Summer Course for Clinical and Translational Research in the Neurosciences, and acknowledges the support from Neurologic AIDS Research Consortium and the Statistical and Data Management Center for the AIDS Clinical Trials Group.
Navigating Regulatory Requirements for Diagnostic Device Trials
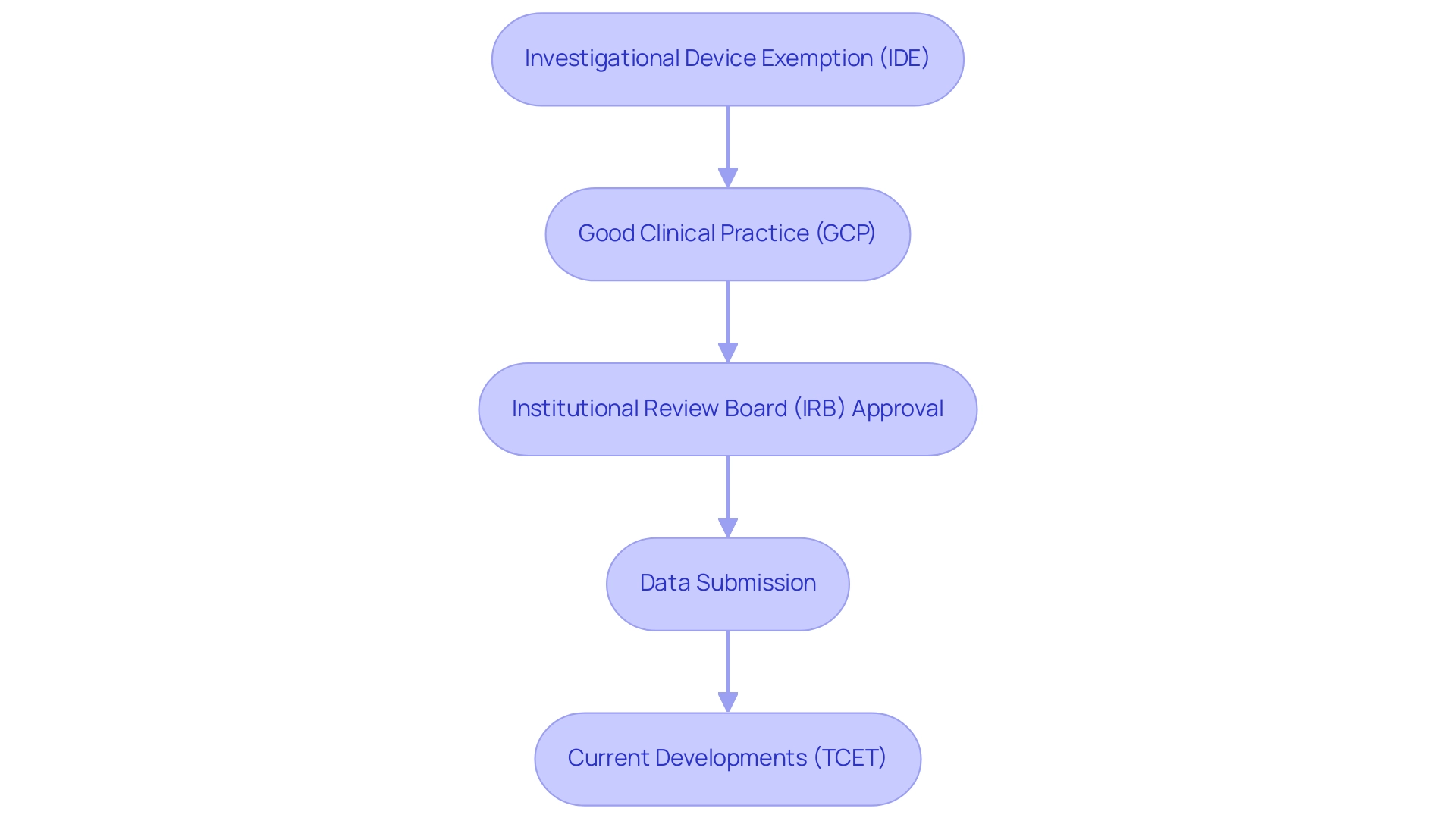
Navigating the regulatory landscape for diagnostic device trials involves several critical steps that ensure compliance and facilitate successful outcomes:
- Investigational Device Exemption (IDE): Securing an IDE from the FDA is a prerequisite for initiating a clinical trial. This exemption permits the use of unapproved devices in research studies, allowing manufacturers to gather essential data on safety and efficacy. Recent statistics indicate that common issues with IDE applications often stem from inadequate reporting of prior investigations, investigational plans, and insufficient detail in design and manufacture descriptions. Addressing these areas can significantly enhance the likelihood of approval. As the FDA states, "The sponsor must demonstrate in the application that there is reason to believe that the risks to human subjects from the proposed investigation are outweighed by the anticipated benefits to subjects and the importance of the knowledge to be gained."
- Good Clinical Practice (GCP): Adherence to GCP guidelines is essential for upholding the ethical and scientific integrity of research studies. Adhering to these standards not only safeguards participant welfare but also ensures that the data collected is reliable and valid. Current compliance statistics highlight that studies demonstrating robust GCP adherence are more likely to achieve favorable outcomes during regulatory reviews.
- Institutional Review Board (IRB) Approval: All clinical research must undergo thorough review and approval by an IRB. This process is essential for ensuring that participant safety and ethical standards are upheld throughout the study. The IRB assesses the project's design, potential risks, and benefits, offering an extra layer of oversight that is vital for ethical research practices.
- Data Submission: Upon completion of the trial, comprehensive data must be submitted to regulatory bodies for evaluation. This submission should clearly demonstrate the device's safety and effectiveness, supported by robust evidence gathered during the study. The FDA provides extensive guidance to assist clinical investigators in understanding the necessary documentation for IDE applications, which can streamline this process and enhance compliance. Utilizing these resources can effectively manage the regulatory process and enhance safety during the examination.
- Current Developments: It is also important to note that the Centers for Medicare & Medicaid Services (CMS) is developing a new Medicare coverage pathway called Transitional Coverage for Emerging Technologies (TCET). This initiative seeks to enable early access to new technologies for Medicare beneficiaries and diminish developer uncertainty concerning coverage, which may influence the regulatory environment for diagnostic assessments.
Comprehending these regulatory requirements is essential for effectively maneuvering through the research process when designing trials for diagnostic devices. By following these steps, manufacturers can mitigate risks and enhance the potential for successful market entry, ultimately contributing to the advancement of innovative medical technologies. Additionally, leveraging comprehensive clinical study management services, such as those provided by bioaccess, can further streamline the process, ensuring compliance and optimizing outcomes.
These services encompass feasibility studies, assessment and feedback on study documents to meet country requirements, setup, initiation, and approval processes, along with reporting on study status, inventory, and serious and non-serious adverse events. With experts like Katherine Ruiz directing regulatory matters for medical products and in vitro diagnostics in Colombia, manufacturers can navigate these complexities with greater confidence.
Defining Objectives and Endpoints for Diagnostic Trials
Establishing clear objectives and endpoints is paramount for the success of diagnostic studies, particularly within the framework of bioaccess®'s comprehensive clinical study management services in Latin America.
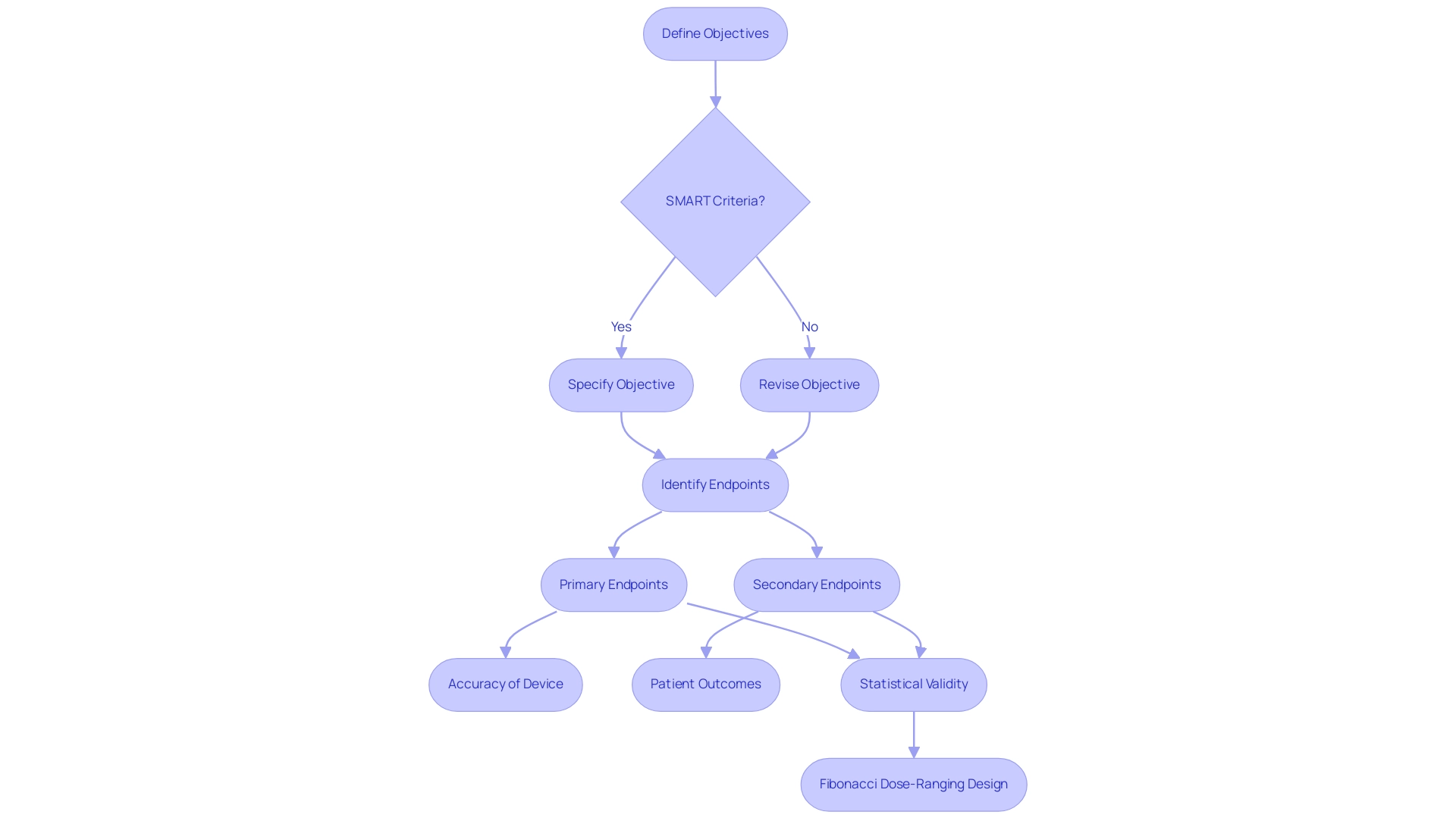
Objectives must adhere to the SMART criteria—specific, measurable, achievable, relevant, and time-bound. For instance, one might articulate an objective as: 'To evaluate the sensitivity and specificity of the diagnostic instrument in detecting disease X.' This clarity not only guides the research but also aligns stakeholders on expected outcomes, a principle that bioaccess® emphasizes in its approach to Early-Feasibility Studies and First-In-Human Studies.
Endpoints encompass both primary and secondary outcomes to be measured throughout the study. Primary endpoints might focus on the accuracy of the device, while secondary endpoints could evaluate patient outcomes or quality of life metrics. The selection of these endpoints is crucial, as they directly influence the study's relevance and applicability in real-world settings. The use of composite endpoints, which combine multiple outcomes, can enhance study efficiency while addressing complexities and potential biases. Careful evaluation and transparent reporting are essential to ensure valid conclusions when utilizing composite endpoints, a practice supported by bioaccess®'s expertise in managing pivotal and post-market clinical follow-up studies.
Statistical considerations are vital to ensure that the endpoints are statistically valid and can be analyzed effectively. For example, employing a Fibonacci dose-ranging design optimizes the allocation of doses across cohorts, enhancing the study's efficiency. In this design, the first cohort receives dose D, the second cohort receives dose 2D, the third cohort receives dose 3D, and the fourth cohort receives dose 5D. This structured method streamlines the process and assists in attaining strong statistical results, aligning with bioaccess®'s dedication to regulatory excellence and innovation in Medtech.
As Ann Yellowlees, Company Founder and Director of Statistics, emphasizes, "Defining clear objectives and endpoints is essential for ensuring that clinical studies yield meaningful and actionable results." By setting clear goals and endpoints, researchers can develop a concentrated and efficient study design, which is essential for designing trials for diagnostic devices in the Medtech field. Bioaccess®'s expertise and customized approach further support this goal, helping to advance medical devices sooner for companies in the Medtech industry.
Selecting the Right Population for Your Clinical Trial
Choosing the appropriate population for your clinical study is a vital step that necessitates careful consideration of several key factors:
- Inclusion Criteria: Clearly define the specific characteristics that participants must possess to be eligible for the study. This may encompass parameters such as age, gender, and health status, which are essential for designing trials for diagnostic devices to ensure that the participant group aligns with the target demographic for the medical device.
- Exclusion Criteria: Identify and articulate the factors that would disqualify potential participants from the research. Common exclusions may involve comorbidities or prior treatments that could obscure the results. Setting these criteria is crucial when designing trials for diagnostic devices, as it helps uphold the integrity of the experiment and ensures that the findings are attributable to the intervention being tested. As emphasized in the Emerge Network's research employing CTKB for electronic phenotyping, designing trials for diagnostic devices through meticulous comparison of eligibility criteria can greatly improve the efficiency and precision of clinical experiments.
- Sample Size Calculation: Precisely determining the required sample size is essential to guarantee that the experiment is sufficiently powered to identify significant differences in outcomes. A well-calculated sample size not only enhances the reliability of the results but also supports the statistical validity of the conclusions drawn from the study. For example, in the context of designing trials for diagnostic devices for Type 2 Diabetes Mellitus, which has seen 5,643 studies, well-defined criteria are crucial for drawing meaningful insights from such a prevalent area of research.
- Designing trials for diagnostic devices requires striving for a diverse participant pool, which is essential for enhancing the applicability of study results. Designing trials for diagnostic devices with a diverse population that reflects the demographics of the intended user base can lead to more generalizable findings, ultimately improving the relevance of the medical device across different populations. Recent analyses indicate that studies with diverse populations yield more robust data, as they account for variations in response to treatment across different demographic groups. As Osarogiagbon observed, the function of oncologists and medical specialists in examining eligibility requirements and sharing insights gained from prior research is essential for guaranteeing the success of trials.
By carefully choosing the participant group, researchers can greatly improve the validity and relevance of their trial outcomes in designing trials for diagnostic devices. This method not only conforms to optimal standards in medical research but also emphasizes the importance of designing trials for diagnostic devices, responding to the increasing focus on inclusivity in health-related investigations, ensuring that the advantages of new medical technologies are reachable for all groups within the population. Additionally, bioaccess™ plays a crucial role in connecting innovative Medtech companies in Latin America, promoting the progress of research studies and ensuring that these best practices are effectively executed.
The partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, seeks to establish Barranquilla as a premier location for medical studies in Latin America, improving the overall environment of health research in the region. bioaccess™ provides a variety of specialized services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, which are crucial for enhancing research outcomes. This partnership has already demonstrated success, achieving over a 50% reduction in recruitment time and maintaining a 95% retention rate, showcasing the effectiveness of their collaborative efforts.
Data Collection and Management Strategies
Implementing robust data gathering and management strategies is crucial for the success of research studies, particularly when designing trials for diagnostic devices within the medical device sector. This section outlines key considerations tailored to navigating the Latin American Medtech landscape:
- Data Collection Methods: The selection of appropriate data collection methods is essential. Options such as electronic case report forms (eCRFs) or traditional paper forms must be evaluated for user-friendliness and efficiency. Current trends indicate that eCRFs are increasingly favored due to their ability to streamline data entry and enhance real-time data access, which is particularly beneficial in diverse markets like Latin America.
- Data Validation: Establishing rigorous data validation processes is vital to ensure the accuracy and completeness of collected data. Techniques such as double data entry and regular audits can significantly reduce errors and enhance data integrity. Industry leaders emphasize that comprehensive reporting, guided by frameworks like SPIRIT and CONSORT, is fundamental for reproducibility and transparency in research. The case study titled 'Importance of Comprehensive Reporting in Research Studies' underscores that clear and open reporting strengthens the completeness and transparency of research reports, ultimately benefiting the scientific community and public understanding.
- Data Security: Protecting sensitive patient information is paramount. Implementing robust security measures that comply with regulations such as HIPAA not only safeguards data but also builds trust with participants. Ensuring that all data handling practices are secure is a critical component of ethical clinical research, especially in regions with varying regulatory environments.
- Data Analysis: Planning for data analysis should commence at the study's inception. This foresight ensures that the collected data can be effectively analyzed to address the research questions posed. Statistical methods must be chosen based on sample sizes, as they influence the calculation of test statistics and p-values, which are crucial for drawing meaningful conclusions. Current statistics indicate that sample sizes significantly affect the methods used for statistical inference, underscoring the importance of careful planning in study design.
By concentrating on these strategies, researchers can significantly enhance the quality and reliability of their study data, which is crucial when designing trials for diagnostic devices. This ultimately contributes to the advancement of medical devices and improving patient outcomes. Moreover, transparent study protocols and impartial participant selection are vital elements that further enhance the integrity of medical research. Leveraging the expertise of bioaccess® can aid these processes, ensuring effective navigation of the intricacies involved in conducting research studies in Latin America.
Monitoring and Quality Control in Clinical Trials
Efficient oversight and quality assurance are paramount for the success of research studies, particularly in the medical device sector. A well-organized approach not only safeguards participant safety but also enhances the reliability of study outcomes. This is especially true when leveraging the comprehensive clinical study management services offered by bioaccess®, which boasts over 20 years of experience in Medtech.
- Monitoring Plans: Crafting a comprehensive monitoring plan is essential. This plan should detail the frequency and techniques of monitoring, ensuring that all elements of the study undergo systematic review. Recent statistics reveal that studies with robust monitoring plans can reduce the risk of protocol deviations by up to 30%, significantly bolstering data integrity. The Geller et al. method, which employs a strong prior, indicates that the number of prior observations should constitute 25% of the cohort size, underscoring the necessity of tailored monitoring strategies.
- Quality Assurance (QA): Implementing rigorous QA processes is critical to ensure compliance with regulatory requirements and adherence to good clinical practices. An analysis highlighted that experiments with established QA protocols experienced a 25% reduction in data discrepancies, reinforcing the importance of these measures. bioaccess® guarantees that all studies meet the requisite compliance reviews and regulatory standards, particularly in the context of INVIMA's oversight in Colombia.
- Data Monitoring Committees: Establishing independent data monitoring committees (DMCs) adds an additional layer of oversight. These committees are tasked with assessing progress and ensuring participant safety, which is particularly vital in research involving high-risk medical devices. The BMT CTN 0601 study exemplifies this, where DMCs effectively monitored safety stopping rules, leading to timely interventions that safeguarded participants. The authors concluded that the methods discussed in this study afford flexibility for investigators in formulating stopping rules with desirable operating characteristics for their research.
- Regular Audits: Conducting regular audits of testing processes and data is essential for promptly identifying and rectifying issues. These audits not only enhance compliance but also foster a culture of continuous improvement within the research team. Establishing a timetable for audits can yield a 20% increase in compliance with study protocols, as evidenced by recent findings in medical research.
As Jimmy T. Le noted, "Specific questions about this Notice should be directed to any member of the NEI Collaborative Clinical Research group at 301-451-2020," emphasizing the importance of expert guidance in developing effective monitoring plans.
By underscoring these facets of oversight and quality assurance, and by leveraging the expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, researchers can significantly enhance the integrity of their studies. This ultimately leads to more dependable and impactful outcomes in the advancement of medical technologies. Furthermore, bioaccess® employs a personalized strategy tailored to meet the specific needs of each client, ensuring optimal results in study management.
Best Practices for Designing Effective Diagnostic Device Trials
Implementing best practices is crucial for significantly enhancing the effectiveness of diagnostic equipment evaluations. This is especially true when leveraging the comprehensive clinical research management services offered by bioaccess®, which boasts over 20 years of experience in the Medtech sector.
-
Clear Protocols: Establishing clear and detailed research protocols is essential. These protocols must comprehensively outline every aspect of the study, from participant recruitment to data analysis, ensuring that all team members are aligned and that the research can be executed smoothly. Designing trials for diagnostic devices necessitates adherence to guidelines established by organizations like the FDA and EMA, guaranteeing their safety and effectiveness.
-
Stakeholder Engagement: Involving key stakeholders, including clinicians and patients, during the study design process is vital. Their insights can ensure that the evaluation remains relevant and feasible, ultimately leading to more meaningful results. Statistics indicate that engaging stakeholders can yield a 99.5% consensus rate on updated design plans, underscoring the importance of teamwork.
-
Pilot Experiments: Conducting pilot experiments is a best practice that allows researchers to assess the practicality of the design. These initial investigations can uncover potential problems and provide an opportunity to make essential modifications before finalizing trials for diagnostic devices, thereby enhancing the overall quality of the research. bioaccess® specializes in managing pilot studies, ensuring they are conducted efficiently and effectively.
Utilizing adaptive study designs is essential when designing trials for diagnostic devices, as it can significantly improve efficiency and outcomes. These designs permit modifications based on interim results, enabling researchers to respond to data as it emerges. Consequently, designing trials for diagnostic devices can yield more robust findings and a better understanding of the diagnostic instrument's performance.
- Statistical Understanding: A fundamental grasp of statistical concepts is vital for conducting and interpreting research effectively. Familiarity with concepts such as the Area Under the Curve (AUC) is crucial for hypothesis testing and confidence interval estimation, which are essential for assessing the performance of diagnostic instruments.
By adhering to these best practices and leveraging the expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies in Latin America, researchers can concentrate on designing trials for diagnostic devices. This focus ultimately increases the likelihood of successful outcomes and advances the development of innovative tools.
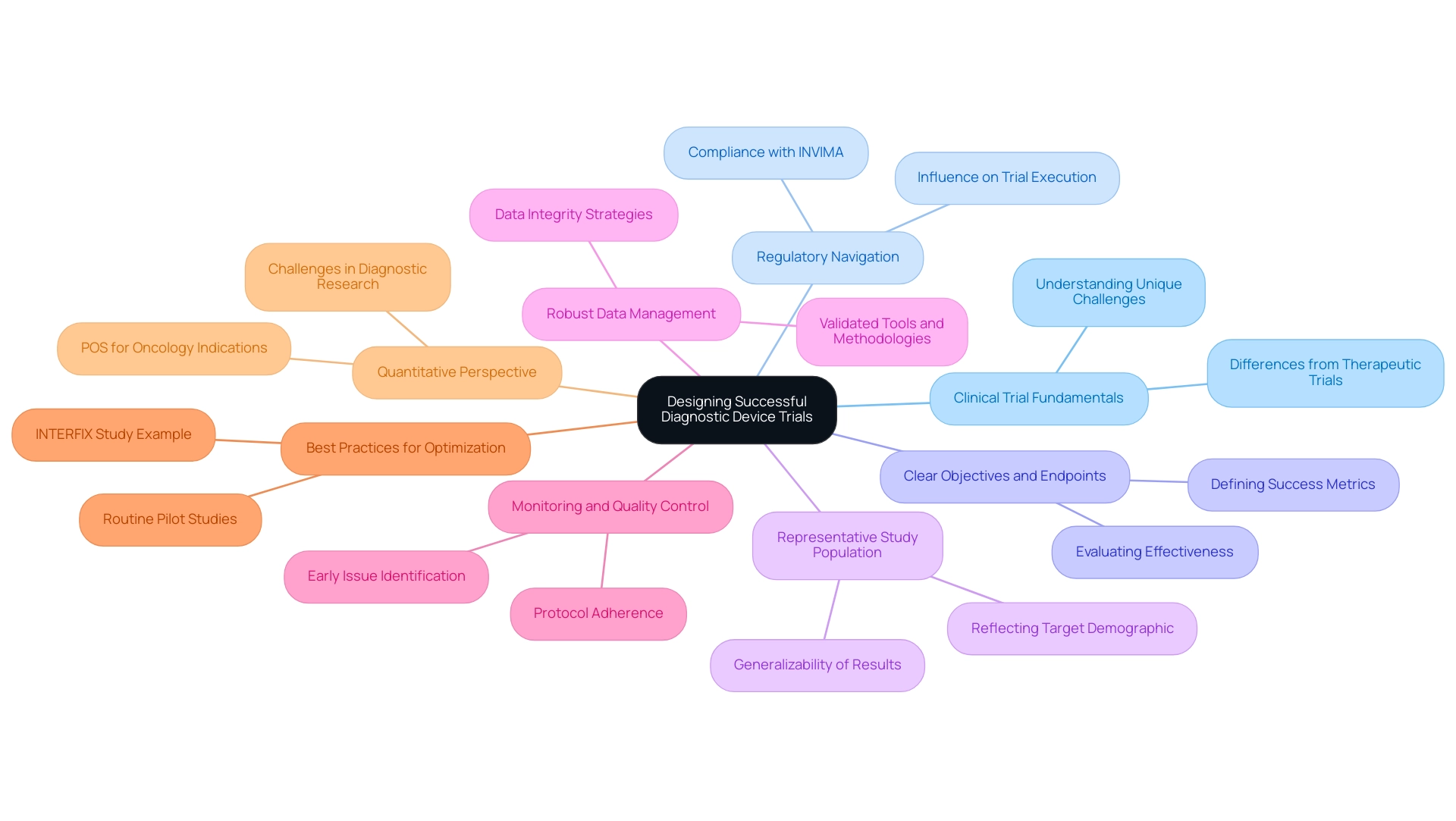
Key Takeaways for Designing Successful Diagnostic Device Trials
Creating efficient assessments for diagnostic tools involves designing trials for diagnostic devices, necessitating a detailed comprehension of research fundamentals and the unique obstacles these instruments pose. Here are the essential takeaways for optimizing trial design:
- Grasp Clinical Trial Fundamentals: A solid foundation in clinical trial design is crucial. This includes acknowledging the unique challenges presented by designing trials for diagnostic devices, which often vary from therapeutic interventions.
- Regulatory Navigation: Careful navigation of regulatory requirements is vital for ensuring compliance and facilitating successful execution. Grasping these regulations can greatly influence the course of the proceedings, especially in Latin America where INVIMA regulates medical equipment.
- Clear Objectives and Endpoints: Defining precise objectives and endpoints is essential for measuring trial success. This clarity helps in evaluating the effectiveness of the diagnostic device under investigation.
- Representative Study Population: Selecting a study population that accurately reflects the target demographic enhances the applicability of results. This step is critical for ensuring that findings can be generalized to broader patient populations.
- Robust Data Management: Implementing strong data collection and management strategies is necessary to maintain data integrity throughout the study. This includes using validated tools and methodologies to gather and analyze data, which aligns with bioaccess®'s commitment to data protection and client trust.
- Monitoring and Quality Control: Prioritizing monitoring and quality control throughout the testing process helps identify issues early and ensures adherence to protocols, ultimately enhancing the reliability of results.
- Best Practices for Optimization: Adhering to recognized best practices in design and execution can result in more efficient and effective research. For instance, routine pilot studies can strengthen study designs and mitigate methodological weaknesses. The INTERFIX study exemplifies this, demonstrating that early measurements can predict health outcomes, justifying early cessation in studies. This approach can enhance the research process in a medical context.
- Quantitative Perspective: It's crucial to acknowledge that the likelihood of success (POS) for lead indications in oncology is merely 11.4%, emphasizing the difficulties encountered in research for diagnostic instruments.
- Building Evidence for Healthcare: As Howard Barkan, an affiliated researcher and consulting statistician, states, "One central goal in conducting methodologically robust studies is to build a sound evidence base for healthcare."
By applying these principles, researchers can significantly improve the quality and effectiveness of their studies, including designing trials for diagnostic devices, ultimately contributing to better patient outcomes and advancing medical technology. Moreover, bioaccess® plays an essential role in connecting Medtech companies in Latin America, enabling the implementation of these best practices in a region full of potential for research. With over 20 years of expertise, bioaccess® ensures that client concerns are addressed with compliance and transparency, reinforcing trust in their comprehensive clinical trial management services.
If you have any queries or concerns about the processing of your information, you may email our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. We will address your concerns in accordance with applicable law. Additionally, bioaccess® specializes in various studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Conclusion
In the intricate landscape of clinical trials for diagnostic devices, a comprehensive understanding of trial design fundamentals is essential for success. Key components, such as well-defined research questions, appropriate study designs, and clearly articulated endpoints, serve as the backbone of effective trials. By meticulously selecting study populations and adhering to regulatory requirements, researchers can enhance the validity and applicability of their findings, ultimately leading to more reliable results.
Challenges such as spectrum and verification bias, alongside regulatory hurdles, necessitate a proactive approach in trial design. Employing best practices—like engaging stakeholders, conducting pilot studies, and utilizing adaptive designs—can significantly improve trial outcomes. Moreover, robust data management and monitoring processes are crucial for maintaining the integrity and reliability of trial data, ensuring participant safety, and fostering trust in the findings.
Ultimately, the pursuit of innovative diagnostic devices hinges on the ability to design and execute trials that not only comply with regulatory frameworks but also yield meaningful and actionable insights. As the Medtech landscape evolves, collaboration with experienced partners, such as bioaccess®, can provide invaluable support in navigating these complexities. By embracing these principles, researchers can contribute to the advancement of medical technology, paving the way for improved patient care and outcomes.
Frequently Asked Questions
What is clinical research design?
Clinical research design is a systematic approach that outlines the methodology for conducting investigations aimed at addressing specific health-related questions.
What are the key components of a robust clinical study framework?
The key components include a well-defined research question, appropriate research design, identification of the target population, and clearly defined endpoints.
Why is the research question important in clinical studies?
The research question serves as the cornerstone of the study, directing the research endeavor and establishing the significance of the outcomes measured.
What types of research designs can be selected for clinical studies?
Research designs include randomized controlled experiments, cohort analyses, and case-control analyses, each suited for different research aims and contexts.
What is the role of endpoints in clinical research?
Endpoints are necessary to evaluate the effectiveness of the diagnostic device, and they should be measurable and relevant to the research question.
How can interim analyses enhance study design?
Interim analyses allow for adjustments in sample sizes and ensure participant safety, typically overseen by an independent Data Safety Monitoring Board (DSMB).
What is the function of a Data Safety Monitoring Board (DSMB)?
The DSMB reviews study data to safeguard participant welfare and assess the efficacy of the study, especially in large or long-duration studies with serious safety concerns.
What challenges are faced when designing trials for diagnostic devices?
Challenges include spectrum bias, verification bias, regulatory hurdles, and patient recruitment issues.
What is spectrum bias in clinical studies?
Spectrum bias occurs when the study population does not accurately reflect the intended use population, leading to skewed and non-generalizable results.
Why is verification bias a concern in clinical research?
Verification bias arises when the reference standard is not uniformly applied across all participants, compromising the validity of the study results.
What is the Investigational Device Exemption (IDE)?
The IDE is a prerequisite from the FDA that permits the use of unapproved devices in clinical trials to gather essential safety and efficacy data.
What are Good Clinical Practice (GCP) guidelines?
GCP guidelines ensure the ethical and scientific integrity of research studies, protecting participant welfare and ensuring reliable data collection.
Why is Institutional Review Board (IRB) approval necessary?
IRB approval ensures that participant safety and ethical standards are upheld throughout the study by assessing the project's design, risks, and benefits.
What steps are involved in the regulatory process for diagnostic device trials?
Key steps include securing an IDE, adhering to GCP, obtaining IRB approval, submitting comprehensive data post-trial, and keeping up with current developments in coverage pathways.
How can partnering with experts assist in navigating regulatory hurdles?
Collaborating with regulatory experts can provide invaluable guidance in overcoming complex requirements, ensuring compliance, and optimizing research outcomes.




