Introduction
The definition and selection of primary endpoints in clinical trials are foundational to the integrity and success of medical research. These endpoints act as the principal measures through which the efficacy of treatments is evaluated, guiding both study design and regulatory scrutiny.
As clinical trials evolve, the emphasis on patient-centered outcomes is reshaping the landscape, prompting researchers to consider not only traditional metrics like overall survival but also quality of life indicators. This shift reflects a deeper understanding of what constitutes meaningful treatment success, as evidenced by recent trends and case studies that illustrate the integration of patient-reported outcomes.
Navigating the complexities of endpoint selection, including the distinction between primary and secondary endpoints, is essential for ensuring that clinical trials yield reliable and impactful results.
Defining Primary Endpoints in Clinical Trials
Main objectives serve as what is a primary endpoint in a clinical study, which is carefully structured to evaluate and plays a crucial role in measuring the effectiveness of a treatment or intervention. These points are characterized by their specificity and quantifiability, reflecting the core objectives of the study. Common examples include overall survival, disease-free survival, and specific biomarker responses.
For example, in clinical studies assessing new cancer medications, the main goal often centers on the overall survival rate of patients over a specified period. The significance of clearly identifying what is a primary endpoint from the beginning cannot be emphasized enough; these objectives are essential for effectively organizing the study and precisely evaluating the outcomes. Significantly, recent statistics show that the P-value for the mind analytical method with three results and an extra result with high incidence and low effect size was <0.03, which emphasizes what is a primary endpoint in the context of clearly defined primary targets.
The growing intricacy and expenses linked to Phase 3 randomized controlled studies highlight the necessity for these measures to effectively showcase treatment effectiveness across various pertinent results. As emphasized by recent studies, including those by Sozu et al., the meticulous approach to defining outcomes is essential for maintaining the integrity and clarity of clinical research results. For R and SAS® codes for implementing the calculations, please see Sozu et al. (2015).
Additionally, several writers have explored techniques for sample size calculation in clinical studies with co-primary objectives, as demonstrated in the case study named 'Sample Size Calculation in CPE Studies,' which stresses the need for larger sample sizes to uphold power when assessing combined effects on multiple objectives.
The Role of Primary Endpoints in Clinical Trial Success
In evaluating the effectiveness of a treatment, understanding what is a primary endpoint is vital, as it serves as the main measure upon which clinical trials are assessed. They are instrumental in shaping the study design and informing the statistical analysis plan. Regulatory bodies, especially the FDA, thoroughly examine results associated with these objectives when considering the approval of new treatments.
Understanding what is a primary endpoint not only clarifies the conclusions regarding a treatment's efficacy but also significantly impacts clinical practice and patient care. For example, when a clinical trial demonstrates a significant advancement in the main outcome compared to a control group, it frequently facilitates the drug's approval and its later incorporation into clinical environments. Recent findings suggest that the anticipated number of cases in Prepare-type data generally varies from 10 to 40, highlighting the significance of strong study designs that concentrate on main outcome results.
This is particularly relevant given the findings of Milligan et al., which demonstrated that fludarabine and cytosine were less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, illustrating what is a primary endpoint and how it can influence treatment decisions. Moreover, a case study on cryotherapy as prophylaxis against oral mucositis illustrates this point; this randomized, open-label, phase 3 study showed that cryotherapy effectively reduced the incidence of oral mucositis in patients undergoing high-dose melphalan and autologous stem cell transplantation, improving comfort during treatment. As pointed out by Dr. Mei-Jie Zhang, the ability to properly analyze results of clinical studies, especially randomized controlled studies (RCT), is a necessary skill for every physician.
This emphasizes the necessity of skillful analysis at the conclusion in facilitating effective treatments and informed regulatory decisions.
Distinguishing Between Primary and Secondary Endpoints
In clinical studies, understanding what is a primary endpoint is crucial as it acts as the key indicator of a trial's success, focusing on the essential results considered most important for assessing treatment effectiveness. Conversely, secondary measures enhance the overall analysis by capturing additional relevant outcomes, which may include quality of life assessments, side effects, or various biomarkers. While main goals, such as overall survival, represent what is a primary endpoint in assessing the main effectiveness of a treatment, secondary measures offer a wider view on the treatment's overall influence.
For example, a trial may prioritize overall survival as its main goal but also assess secondary objectives such as progression-free survival and adverse effects. This dual approach allows researchers to understand what is a primary endpoint, as well as both the efficacy and safety of a treatment. It is crucial to recognize that secondary outcomes, although significant, do not possess the same statistical weight as main outcomes, making favorable changes in secondary outcomes more likely due to randomness.
For example, if 100 different non-pre-specified subgroups are evaluated, we would expect five to have a p-value < 0.05, highlighting the risk of chance findings in secondary analyses. As highlighted in the case study titled 'Statistical Authority of Endpoints,' secondary outcomes primarily serve to interpret primary results or guide future research rather than to draw definitive conclusions. Additionally, the analysis of survival by tumor response discussed by Anderson et al.
exemplifies the practical use of these interfaces. This nuanced understanding emphasizes the importance of carefully designing trials that consider both types of outcomes, especially in light of current discussions on their significance and the biases inherent in analyses comparing responders and non-responders. As Robert Peter Gale aptly states, 'the insights gained from secondary measures are invaluable but must be interpreted with caution to avoid misleading conclusions.
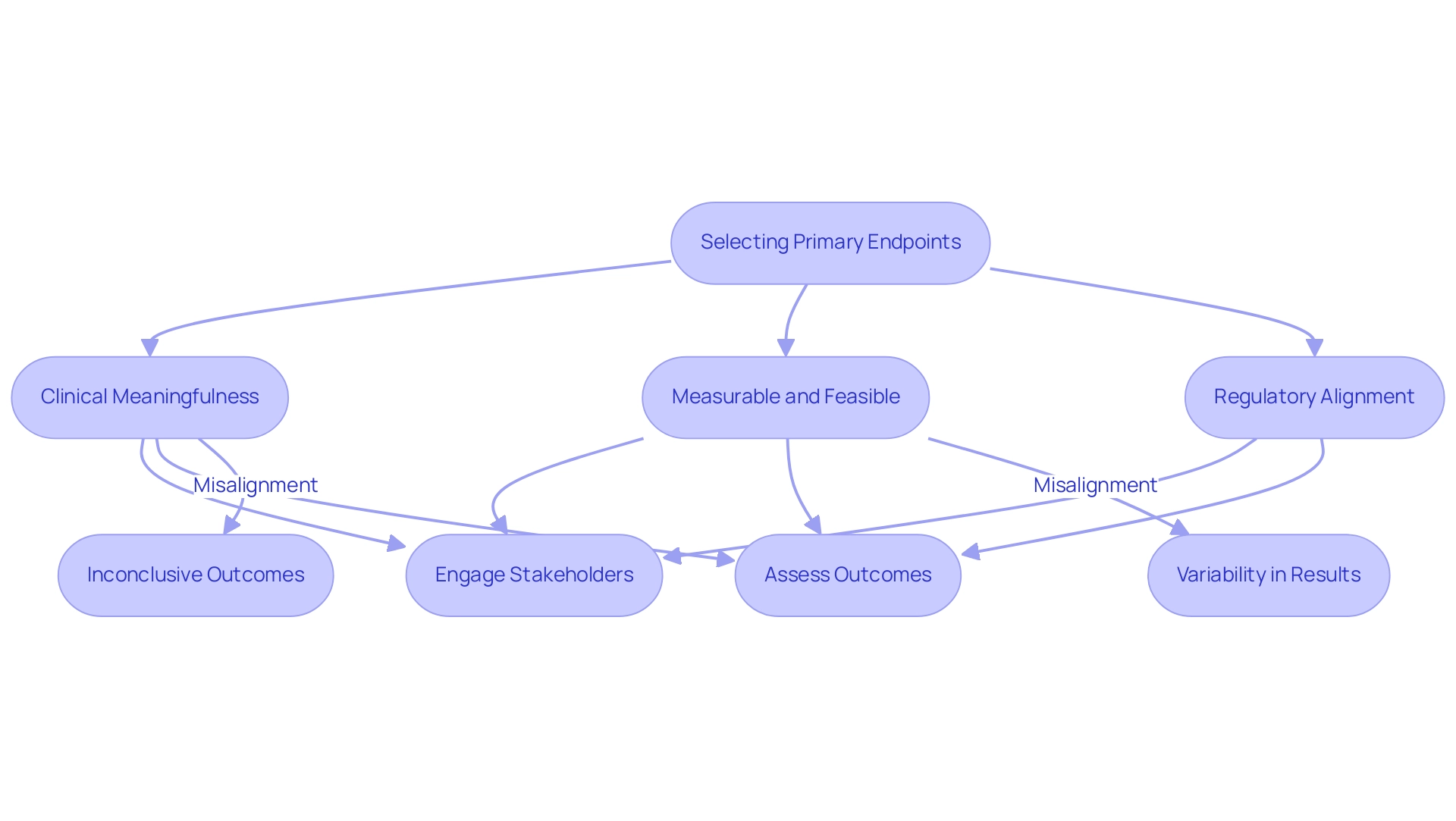
Challenges in Selecting Primary Endpoints
Researchers face several challenges in choosing a suitable main goal, which raises the question of what is a primary endpoint. It is imperative to understand what is a primary endpoint, ensuring that the selected target is not only clinically meaningful but also measurable and feasible within the framework of the study's design. Aligning the target with regulatory expectations is crucial, as it must possess the sensitivity to detect what is a primary endpoint related to any treatment effects that may arise.
For example, Carvedilol has been proven to lower mortality by 65%, emphasizing the importance of choosing pertinent measures that can illustrate significant results. Misalignment in selection of targets can lead to inconclusive outcomes and, ultimately, unsuccessful experiments. For instance, choosing a target that is excessively broad or subjective can introduce variability in results, complicating data interpretation significantly.
As noted by Sebastien Coppe, CEO of One2Treat, involving multiple patient-relevant outcomes in the clinical study design can help consolidate both efficacy and safety results into a single primary metric, prompting the discussion on what is a primary endpoint and mitigating the challenges associated with non-inferiority studies. This highlights the significance of comprehensive discussions among stakeholders, including biostatisticians, clinicians, and regulatory experts, to enable informed decision-making in outcome selection. Additionally, the development of outcome categories, as demonstrated in the case study titled 'Evolution of Endpoints,' highlights a growing awareness of the complex impacts of interventions and emphasizes the need for personalized measures that assess individually defined risks and advantages in patient-focused healthcare in clinical research.
Recent Trends and Examples of Primary Endpoints in Clinical Trials
The setting of clinical studies is increasingly emphasizing patient-focused results as main objectives, indicating a notable advancement in study design. In oncology, while conventional metrics such as overall survival and progression-free survival remain significant, there is a notable shift towards incorporating quality of life measures as crucial main goals. A notable illustration of this trend can be seen in evaluations of new cancer treatments, where the emphasis has broadened to incorporate patient-reported results.
Here, what is a primary endpoint has emerged as the improvement in quality of life, rather than solely relying on survival metrics. This shift signifies a broader understanding of treatment success, recognizing that clinical efficacy must be complemented by considerations of the patient's overall experience and well-being. For instance, the ESETT study, which randomized participants to three intravenous anticonvulsive agents, illustrates a concrete example of research design changes aimed at enhancing patient-centered results.
Furthermore, CardioCube has displayed encouraging outcomes in a feasibility study involving cardiovascular patients, demonstrating an impressive 97.5 percent accuracy rate in gathering cardiovascular risk factors and past medical history, highlighting the effectiveness of patient-centered methods in clinical research. Furthermore, the case study titled 'Adapting Multiple Features' highlights how utilizing various adaptive design elements can enhance patient-centered results, providing flexibility and appeal to participants while acknowledging the increased complexity and required statistical work. Such an approach not only enhances the relevance of trial outcomes but also aligns with the growing emphasis on patient-centered care in the healthcare landscape for 2024.
Conclusion
The selection of primary endpoints in clinical trials is crucial for the integrity and success of medical research. These endpoints serve as key measures for evaluating treatment efficacy and play a vital role in shaping study design and regulatory evaluations. The growing emphasis on patient-centered outcomes signifies a shift in how endpoints are defined, with an increasing focus on incorporating quality of life indicators alongside traditional metrics.
Understanding the distinction between primary and secondary endpoints is essential. Primary endpoints assess core treatment efficacy, while secondary endpoints provide additional insights into safety and quality of life. This comprehensive approach ensures that all relevant outcomes are considered in evaluating new therapies.
However, selecting appropriate primary endpoints presents challenges, requiring careful consideration to ensure they are clinically meaningful, measurable, and aligned with regulatory expectations. Engaging stakeholders in this process is critical to avoid the risks associated with poorly defined endpoints, which can lead to inconclusive results.
As the focus on patient-centered care continues to evolve, integrating patient-reported outcomes as primary endpoints will enhance the relevance of clinical trials. This shift reflects a deeper understanding of treatment success and emphasizes the need for methodologies that prioritize patient experiences. A thoughtful approach to endpoint selection is essential for ensuring clinical trials produce reliable results that contribute to improved patient outcomes and informed healthcare decisions.
Frequently Asked Questions
What is a primary endpoint in a clinical study?
A primary endpoint is a specific and quantifiable measure used to evaluate the effectiveness of a treatment or intervention in a clinical study. It serves as the main objective that shapes the study design and informs the statistical analysis plan.
Why are primary endpoints important?
Primary endpoints are crucial for measuring treatment effectiveness, organizing the study, and evaluating outcomes. They are closely examined by regulatory bodies, such as the FDA, when considering the approval of new treatments.
What are common examples of primary endpoints?
Common examples of primary endpoints include overall survival, disease-free survival, and specific biomarker responses.
How do primary endpoints influence clinical practice?
When a clinical trial demonstrates a significant advancement in the primary endpoint compared to a control group, it can facilitate the drug's approval and its later incorporation into clinical practice, impacting patient care.
What challenges are associated with defining primary endpoints?
The complexities and costs of Phase 3 randomized controlled studies necessitate well-defined primary endpoints to effectively showcase treatment effectiveness across various outcomes.
How does sample size relate to primary endpoints?
Larger sample sizes are often required to maintain statistical power when assessing combined effects on multiple primary endpoints, as indicated by various studies on sample size calculation in clinical research.
Can you provide an example of a study that illustrates the importance of primary endpoints?
A case study on cryotherapy as prophylaxis against oral mucositis demonstrated that it effectively reduced the incidence of oral mucositis in patients undergoing high-dose melphalan and autologous stem cell transplantation, highlighting the role of primary endpoints in influencing treatment decisions.
What recent findings emphasize the significance of primary endpoints in clinical studies?
Recent findings suggest that the anticipated number of cases in Prepare-type data varies from 10 to 40, underscoring the importance of strong study designs that focus on primary outcome results.




