Overview
The article emphasizes the remarkable growth of clinical research infrastructure in Latin America, underlining its emergence as a competitive hub for medical studies. This development is attributed to advancements in facilities, regulatory support, and patient engagement strategies. Notably, the establishment of state-of-the-art research centers in Brazil, Mexico, and Colombia exemplifies this trend.
Legislative reforms further streamline processes and enhance recruitment, ultimately positioning the region for future innovation and collaboration in medical research.
Introduction
In recent years, Latin America has emerged as a dynamic force in the realm of clinical research, showcasing a remarkable evolution in its infrastructure and capabilities. Major cities in Brazil, Mexico, and Argentina have established themselves as key players, equipped with state-of-the-art facilities and a workforce of highly skilled professionals. This transformation transcends mere technological advancements; it reflects a broader commitment to enhancing health equity and addressing local health priorities.
As the region embraces decentralized clinical trials and innovative methodologies, it positions itself as an attractive destination for researchers and companies seeking efficient and cost-effective solutions. With a focus on patient-centric approaches and robust regulatory support, Latin America is poised to redefine the landscape of global clinical research, offering diverse opportunities that promise to advance medical science and improve healthcare outcomes.
Overview of Clinical Research Infrastructure in Latin America
Over the past decade, the growth of infrastructure in Latin America has significantly enhanced its medical investigation framework, establishing the region as an emerging hub for medical studies. Major cities throughout Latin America, particularly in Brazil, Mexico, and Argentina, now boast cutting-edge facilities that meet international standards, underscoring the region's infrastructural advancements. These centers are equipped with state-of-the-art technology and staffed by highly skilled professionals, enabling a diverse array of research studies, ranging from early-phase investigations to post-market assessments.
By 2025, the number of medical study facilities in Brazil, Mexico, and Argentina has seen a remarkable increase, reflecting a strong commitment to enhancing research capabilities. For instance, Brazil has enacted Law 14.874/24, which streamlines the evaluation process for research studies, thereby accelerating the initiation of investigations. This legislative progress is coupled with an increasing focus on health equity and regional health priorities, ensuring that medical research is not only innovative but also responsive to the needs of local populations.
Colombia has emerged as a premier destination for first-in-human (FIH) clinical studies, offering significant competitive advantages. The country provides cost reductions exceeding 30% compared to studies in North America or Western Europe, while the comprehensive IRB/EC and MoH (INVIMA) review process takes only 90-120 days. The World Health Organization ranks Colombia's healthcare system as #22 globally, with its hospitals recognized among the finest in the Americas, ensuring high-quality care for trial participants.
With a population surpassing 50 million and 95% coverage under universal healthcare, patient recruitment is robust, further enhancing Colombia's appeal for research studies. Additionally, investments in science, technology, and innovation projects in Colombia benefit from a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants, making it financially attractive for medical device companies.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked, "My experience with bioaccess® during the initial human study in Colombia illustrated the effectiveness of local CROs in navigating the regulatory framework." This insight emphasizes the value of local expertise, particularly from bioaccess®, which specializes in providing cost-effective, high-quality medical device CRO services in Latin America. Their extensive offerings include regulatory approval, site activation for studies, participant recruitment, and data management, facilitating expedited study outcomes.
The collaboration between public and private sectors has been essential in fostering an environment that promotes innovation and efficiency, particularly in the context of infrastructure growth in Latin America trials in medical studies. This synergy has led to the emergence of decentralized studies and the integration of digital technologies, contributing to the region's infrastructural advancements and transforming the landscape of medical exploration. Furthermore, the emphasis on ethics and patient protection remains paramount, ensuring that the rights and well-being of participants are safeguarded throughout the research process.
Hospitals in Colombia are authorized to conduct trials with pharmaceutical drugs only after undergoing a rigorous ICH/GCP certification process, ensuring quality assurance in studies.
As South America advances its medical study framework, the infrastructure growth in Latin America trials positions the region to emerge as a significant contributor to global medical inquiries, attracting both local and international partnerships. The ongoing development of study facilities not only supports the advancement of medical technologies but also enhances healthcare outcomes across the region. Additionally, Chile is emerging as a promising market for medical study tourism, particularly in the realm of rare diseases and academic research, further expanding the opportunities within the region's research landscape.

Benefits of Conducting Clinical Trials in Latin America
Carrying out clinical studies in South America offers numerous benefits that render it an appealing choice for researchers and businesses alike.
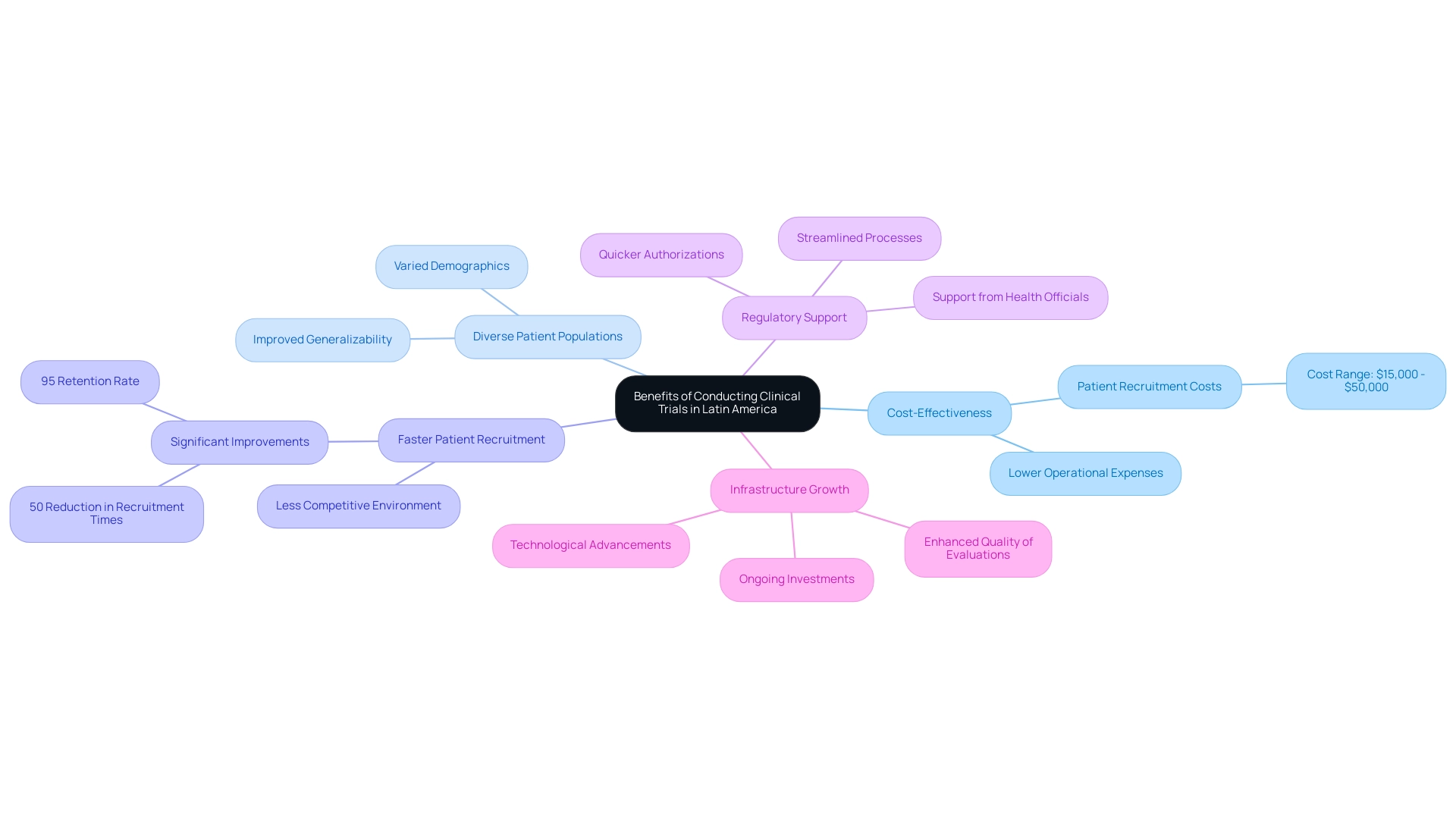
- Cost-Effectiveness: The operational expenses in South America are considerably lower than those in North America and Europe, with patient recruitment costs varying from $15,000 to $50,000. This cost efficiency permits more budget-conscious experiments, enabling companies to allocate resources more effectively.
- Diverse Patient Populations: South America boasts a rich tapestry of demographics, providing access to varied patient populations. This diversity improves the generalizability of study results, making findings more relevant across various regions and populations.
- Faster Patient Recruitment: The competitive environment for patient enrollment in South America is less intense compared to North America and Europe. This leads to faster enrollment periods, enabling studies to advance more swiftly and effectively. For instance, the collaboration between bioaccess™ and Caribbean Health Group has led to significant improvements in recruitment times, achieving over a 50% reduction and maintaining a remarkable 95% retention rate.
- Regulatory Support: Many countries in Latin America have made strides in streamlining their regulatory processes. This proactive method enables quicker authorizations for research studies, shortening the duration from project conception to commencement. The backing from Colombia's Minister of Health further highlights the dedication to improving the medical investigation environment in the area.
Ongoing investment in study facilities and technological advancements is enhancing the quality and efficiency of medical evaluations in the region, reflecting the importance of infrastructure growth in Latin America trials. This expansion in infrastructure growth in Latin America trials aids the rising need for high-quality medical study services. Significantly, bioaccess™ serves as a Functional Service Provider (FSP) for biotechnology and pharmaceutical firms, improving their operational abilities and enabling them to carry out experiments more efficiently across diverse areas.
The combination of these elements not only establishes South America as a feasible choice for experimental studies but also highlights the region's capacity to facilitate the swift progress of medical technologies. As emphasized in recent discussions, the lower dropout rates in Latin America—attributed to strong patient-physician relationships and urban subject concentrations—further enhance the region's attractiveness for conducting medical research. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, emphasizing the effectiveness of these partnerships.
With the appropriate strategies and partnerships, companies can utilize these advantages to speed up their research studies and bring innovative medical devices to market more swiftly. The collaboration was announced on March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, which was attended by Colombia's Minister of Health.
Emerging Trends: Decentralized Clinical Trials in Latin America
Decentralized studies (DCTs) are swiftly transforming the landscape of medical research in Latin America, propelled by technological advancements and a strong focus on patient-centered methodologies. The key attributes of DCTs encompass:
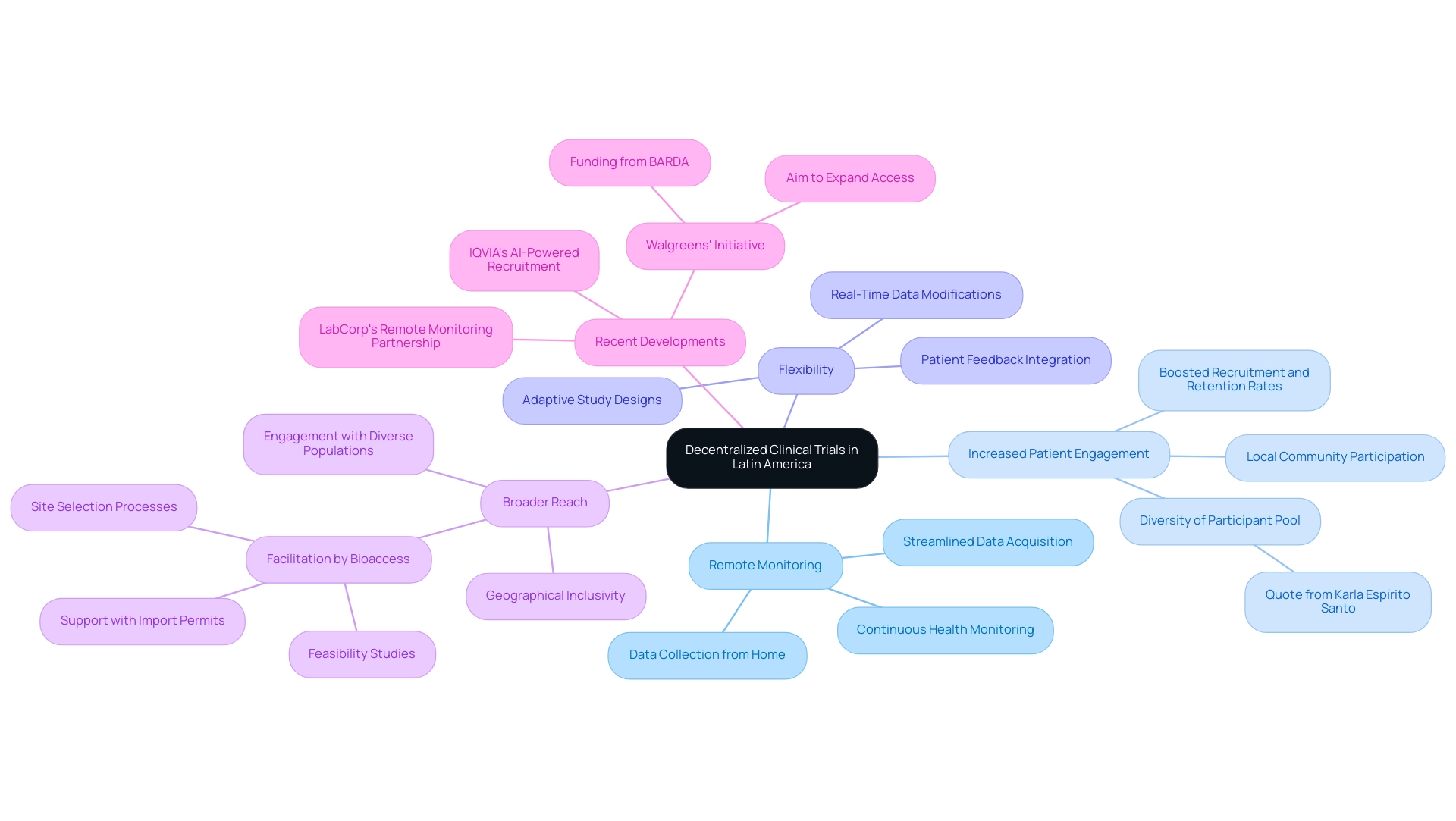
- Remote Monitoring: By leveraging digital technologies, DCTs enable continuous monitoring of patient health and data collection from the comfort of their homes, significantly reducing the need for in-person site visits. This approach not only enhances patient convenience but also streamlines data acquisition processes, aligning with bioaccess's commitment to comprehensive clinical study management services, including compliance reviews and reporting.
- Increased Patient Engagement: DCTs facilitate patient participation in studies within their local communities, effectively boosting recruitment and retention rates. This localized approach is vital for cultivating a diverse participant pool, essential for the generalizability of study results. As Karla Espirito Santo, leader of Decentralized Studies and New Clinical Experiment Models at Hospital Israelita Albert Einstein’s ARO, articulates, "The idea is to increase the diversity of participants in studies, and not just among the small group of patients already attending medical centers."
- Flexibility: Characterized by adaptive study designs, DCTs can be modified in response to real-time data and patient feedback. This flexibility is crucial for addressing the dynamic nature of medical studies and ensuring evaluations remain relevant and efficient, a principle that bioaccess embodies in its project management and monitoring services.
- Broader Reach: The decentralized model empowers researchers to engage with diverse populations across extensive geographical areas, thereby enhancing the inclusivity of studies. This is particularly significant in South America, where access to medical research may be limited in certain regions. Bioaccess plays a pivotal role in facilitating this reach through its feasibility studies, site selection processes, and support with import permits and nationalization of investigational devices.
The expansion of DCTs in Latin American trials is underscored by substantial investments and initiatives aimed at bolstering infrastructure to enhance access to research studies. For instance, Walgreens' recent initiative, supported by a $25 million investment from the Biomedical Advanced Research and Development Authority (BARDA), strives to improve participation in decentralized research studies, addressing barriers to access and fostering broader involvement. This funding is expected to facilitate greater participation in medical studies, tackling obstacles to access and supporting infrastructure growth in Latin America trials to enhance the scope of decentralized health investigations.
Moreover, recent advancements such as IQVIA's introduction of an AI-driven patient recruitment platform and LabCorp's partnership with a digital health firm for remote monitoring further illustrate the progress in decentralized studies, enhancing the timeliness and significance of this approach.
As the landscape of medical studies continues to evolve, the emphasis on patient-centered methods and the integration of technology will be pivotal in shaping the future of healthcare evaluations in South America. The trends observed in 2025 indicate a promising trajectory for DCTs, highlighting the enhancement of patient involvement and ensuring that studies are accessible to a wider audience, supported by organizations like bioaccess that excel in facilitating medical device evaluations in the region.
The Role of CROs in Supporting Clinical Trials
Contract Research Organizations (CROs) play a pivotal role in the clinical study landscape of Latin America, offering a spectrum of services that significantly enhance the efficiency and effectiveness of research initiatives. Their contributions can be categorized as follows:
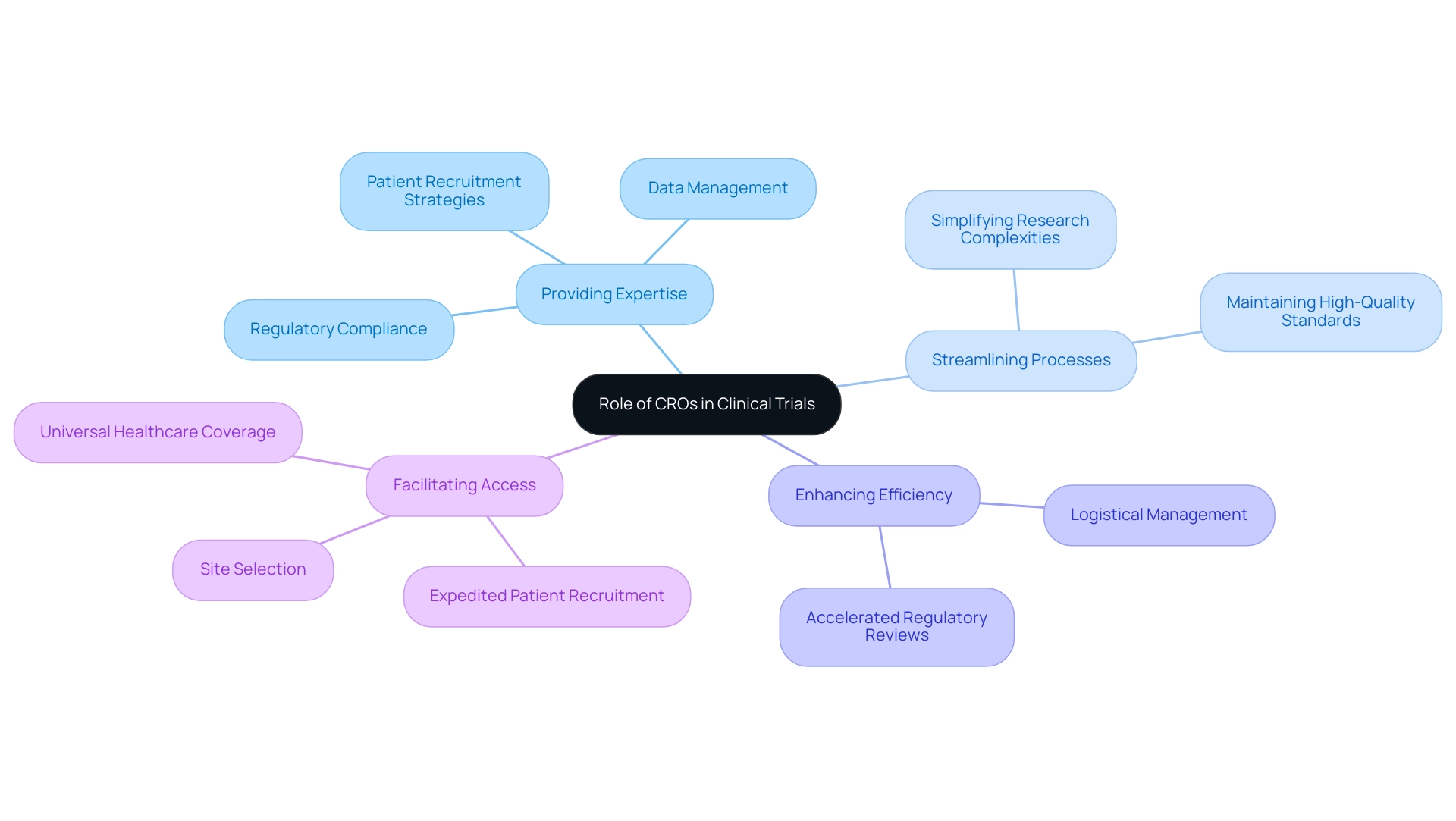
- Providing Expertise: CROs possess specialized knowledge essential for navigating the intricate web of regulatory compliance, patient recruitment, and data management. This expertise is particularly vital in a region where regulatory frameworks can vary widely, ensuring that studies adhere to both local and international standards. Notably, the INVIMA is classified as a Level 4 health authority by PAHO/WHO, underscoring the importance of CROs in assisting Medtech companies with these complex regulations. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, emphasized the critical role of bioaccess® during its initial human study in Colombia, illustrating how local CROs can facilitate compliance and efficiency. His experience demonstrates that bioaccess® not only simplified the regulatory process but also ensured adherence to high-quality standards, which is crucial for achieving successful outcomes.
- Streamlining Processes: By simplifying the complexities associated with research studies, CROs ensure that investigations comply with necessary regulations while maintaining high-quality standards. This streamlining is essential for Medtech companies aiming to accelerate their product development timelines. Colombia's healthcare system, recognized among the best globally, supports this effort by providing a robust environment for medical research.
- Enhancing Efficiency: CROs take on the logistical and administrative responsibilities of research studies, including monitoring and reporting. This delegation allows researchers to focus on the scientific aspects of their work, ultimately leading to more innovative outcomes. The speed of regulatory reviews in Colombia, averaging 90-120 days, further enhances this efficiency, making it an attractive destination for first-in-human studies.
- Facilitating Access: With established networks and relationships within the healthcare ecosystem, CROs can expedite patient recruitment and site selection. This capability is crucial for timely study completion, particularly in a diverse region like Latin America, where access to varied patient populations can significantly impact timelines. Colombia's universal healthcare coverage for approximately 95% of its population offers a substantial pool for patient recruitment, further supported by R&D tax incentives that encourage investment in research studies.
Bioaccess® specializes in various study types, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies. These services highlight bioaccess®'s capabilities as a CRO, reinforcing its value proposition in facilitating research studies.
The evolution of the CRO market in South America is noteworthy, with forecasts indicating a robust increase driven by the rising demand for studies in the Medtech sector, closely linked to infrastructure growth in Latin American trials. Collaborating with local CROs not only provides strategic advantages, such as reduced study expenses and expedited patient enrollment, but also enhances the overall quality of research. As emphasized by industry specialists, including Julio G. Martinez-Clark, the CEO of bioaccess®, "The government of Colombia appears to be the only nation in South America with a proactive initiative to attract more research studies as part of its strategy to transform into a knowledge economy by 2031." This statement underscores regional initiatives to improve research opportunities and supports the narrative regarding the significance of CROs.
Case studies illustrate the successful partnerships formed between Medtech companies and CROs in the region. For instance, the findings from the case study titled "Conclusion on the Role of American CROs" underscore how these collaborations have led to efficient trial execution and groundbreaking medical advancements, showcasing specific outcomes that exemplify the effectiveness of CROs in facilitating research trials in the region. As the infrastructure growth in Latin American trials and the technological capabilities of South American CROs continue to evolve, their role in the global research landscape is poised to become even more significant.
Navigating the Regulatory Landscape of Clinical Research
Navigating the regulatory landscape in South America presents unique challenges that require a nuanced understanding of local laws and guidelines. Key considerations include:
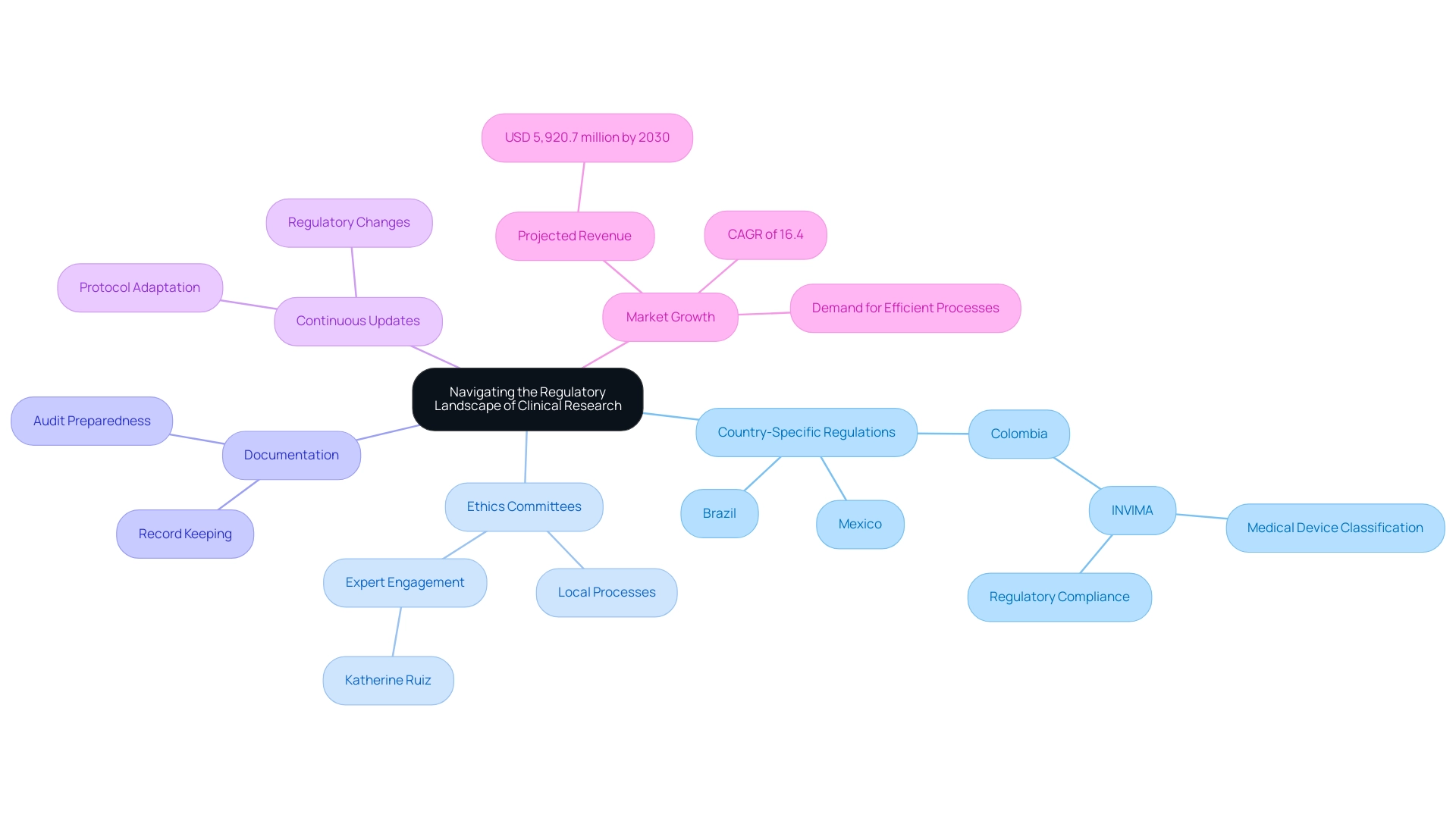
- Country-Specific Regulations: Each nation in Latin America operates under its own regulatory body, establishing distinct requirements for clinical trials. This necessitates a tailored approach for each study, as regulations can vary significantly between countries such as Brazil, Mexico, and Colombia. For example, Colombia's INVIMA (National Food and Drug Surveillance Institute) functions as a Level 4 health authority, managing medical device classification and regulatory compliance, which is essential for successful execution.
- Ethics Committees: Obtaining consent from local ethics committees is an essential step in the research process. Familiarity with the specific processes and expectations of these committees can significantly expedite approvals, thereby reducing time to market for innovative medical devices. Engaging with experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices in Colombia, can provide invaluable insights into navigating these approvals, further enhancing bioaccess®'s customized approach to regulatory navigation.
- Documentation: Maintaining meticulous records and comprehensive documentation is essential for compliance with regulatory standards. This diligence not only facilitates smoother audits and inspections but also enhances the overall integrity of the research process. bioaccess® highlights the significance of detailed documentation in its extensive study management services, which encompass feasibility studies, setup, and project oversight, supported by over 20 years of experience in Medtech.
- Continuous Updates: The regulatory environment is dynamic, with frequent updates and changes. Staying informed about these developments is crucial for ensuring ongoing compliance and adapting protocols as necessary. bioaccess®'s proficiency in early feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies establishes it as a prominent contract research organization in facilitating medical device studies in South America.
The research study technology and services market in Latin America is anticipated to attain USD 5,920.7 million by 2030, indicating a compound annual growth rate (CAGR) of 16.4% from 2025 to 2030. This growth highlights the rising need for effective testing processes and enhanced research infrastructure, which are essential components of infrastructure growth in Latin America trials, positioning the region as a major participant in the global market. Effective navigation of the regulatory environment is essential not just for compliance but also for utilizing the competitive benefits that nations like Colombia provide, including cost efficiency and swift procedures for initial human studies.
Incorporating data visualization tools has emerged as a best practice to enhance decision-making in research studies, particularly in countries like Brazil, Mexico, and Argentina. This method underscores the significance of continuous observation and flexibility in medical studies, ensuring that evaluations stay in accordance with regulatory standards and market demands. Significantly, GlobalCare Clinical Trials' partnership with bioaccess® has resulted in a decrease of over 50% in recruitment time and 95% retention rates, demonstrating the effectiveness of bioaccess®'s services in enhancing study efficiency.
As Nuray Kenzhebek, a marketing specialist, observes, "The increasing significance of South America in FDA-regulated studies emphasizes a wider trend: the globalization of medical research." This context further highlights the importance of the area in the changing environment of medical studies.
Challenges in Clinical Research: Recruitment and Operational Hurdles
Researchers in Latin America frequently encounter a range of challenges that can impede the success of clinical trials.
- Patient Recruitment: While the region boasts a diverse population, effectively reaching potential participants remains a significant hurdle. Cultural nuances and socioeconomic disparities complicate recruitment efforts, necessitating tailored strategies to engage various communities. Notably, with a literacy rate of 95.7% in Chile, understanding local dynamics is crucial for optimizing these strategies. Successful collaborations, such as that of GlobalCare Clinical Trials with bioaccess®, demonstrate the effectiveness of leveraging local knowledge to reduce research participant recruitment duration by over 50% while achieving a participant retention rate exceeding 95%.
- Operational Hurdles: Logistical challenges, including transportation difficulties and communication barriers, can disrupt the flow of experiments. These issues often require innovative solutions, such as utilizing technology to streamline processes and enhance coordination among stakeholders. bioaccess®'s comprehensive clinical study management services, encompassing setup and project management, are designed to effectively address these operational hurdles.
- Regulatory Delays: The complex regulatory landscape in Latin America can lead to unforeseen delays if not navigated carefully. Establishing strong relationships with local regulatory authorities is crucial for ensuring compliance and expediting approvals. A local presence and leadership, exemplified by bioaccess® in Colombia, can significantly enhance these relationships and facilitate smoother processes.
- Resource Limitations: Certain areas may experience insufficient resources or infrastructure, negatively impacting the quality and efficiency of clinical studies. Addressing these limitations often involves collaboration with experienced contract research organizations (CROs) that understand the local context and can provide necessary support for infrastructure growth in Latin America trials. bioaccess® serves as a vetted CRO and consulting partner for U.S. medical device firms, ensuring that studies are conducted efficiently and effectively.
To mitigate these challenges, strategies such as engaging local communities, utilizing technology for outreach, and fostering partnerships with established CROs can prove highly effective. The recent increase in funding for research studies across the Andean Region—from $3-4 million to over $50 million each year—highlights the growing acknowledgment of these challenges and the necessity for creative solutions, particularly regarding infrastructure growth in Latin America trials. By overcoming linguistic, cultural, and socio-economic obstacles, sponsors can enhance patient safety and improve health outcomes, ultimately advancing the field of medical technology in South America.
As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, stated, "I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals."
Additionally, bioaccess® specializes in various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® is well-equipped to handle the intricacies of medical studies, contributing to job creation, economic growth, and healthcare enhancement in the region.
Future Perspectives: Growth and Innovation in Clinical Research
The future of clinical research in Latin America is poised for remarkable growth and innovation, propelled by several key factors:
- Technological Advancements: The integration of artificial intelligence and machine learning is revolutionizing trial design and data analysis, significantly enhancing both efficiency and accuracy. These technologies empower researchers to optimize procedures, reduce costs, and improve outcomes, making studies more effective than ever.
- Increased Funding: As Latin America gains recognition for its robust investigative capabilities, investment in health studies is expected to surge. This influx of capital will bolster infrastructure development in Latin American trials, facilitating the establishment of state-of-the-art facilities and resources that advance clinical research initiatives.
- Patient-Centric Approaches: A strong focus on patient engagement and the implementation of decentralized research models are anticipated to enhance recruitment and retention rates. By prioritizing the patient experience, researchers can ensure that studies are more accessible and better aligned with the needs of diverse populations.
- Regulatory Harmonization: Ongoing efforts to align regulations across South American nations will streamline the testing process, making it more attractive for global sponsors. This alignment not only enables smoother operations but also enhances the region's competitiveness in the global health study landscape.
As highlighted in the Horizon Databook, the infrastructure development in Latin American trials is rapidly evolving within the research trial technology and services market, with increasing demand for efficient trial processes. The databook indicates that the market is projected to grow significantly, driven by technological advancements and a favorable investment climate. This growth is further supported by beneficial policies, such as Colombia's 100% tax deduction for investments in science, technology, and innovation projects, which fosters development and progress.
Moreover, the emphasis on health equity and access to care is inspiring initiatives that address local health challenges, ensuring that medical studies are not only innovative but also inclusive.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked, "My experience with bioaccess® during the initial human study in Colombia illustrated the effectiveness of local CROs in navigating the regulatory framework." This insight underscores the benefits of collaborating with local organizations like bioaccess®, which possess in-depth knowledge of the regional landscape and specialize in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
In summary, the convergence of technological advancements, increased investment, patient-centric strategies, and regulatory improvements bolsters the infrastructure growth in Latin American trials, positioning South America as a burgeoning hub for medical studies and promising a future rich with opportunities for innovation and collaboration. Additionally, bioaccess® has published best practices and strategies for conducting successful innovative clinical research trials in Latin America, further demonstrating its commitment to advancing the field.

Conclusion
The evolution of clinical research in Latin America is significantly impacting the global medical landscape. With state-of-the-art facilities in Brazil, Mexico, and Argentina, the region is enhancing its infrastructure while focusing on health equity and addressing local health priorities. The rise of decentralized clinical trials and patient-centric methodologies makes Latin America an appealing destination for researchers and companies seeking efficient, cost-effective solutions.
Key benefits of conducting clinical trials in this region include:
- Lower costs
- Diverse patient populations
- Quicker recruitment
- Supportive regulatory environments
Local expertise from Contract Research Organizations (CROs) is essential for navigating complex regulations, which contributes to successful trial outcomes.
The trend towards decentralized trials enhances patient engagement and broadens access, ensuring diversity in participant demographics. Collaboration between public and private sectors fosters an innovative ecosystem, reinforcing Latin America's role in global clinical research.
Looking forward, the future of clinical research in Latin America is bright, driven by ongoing investments, regulatory harmonization, and a commitment to patient-centered approaches. The region is poised to become a key player in advancing medical science and improving healthcare outcomes, offering unique opportunities for innovation and collaboration. As this landscape evolves, local organizations will be crucial in navigating the complexities of clinical trials, ensuring that advancements in medical technology benefit diverse communities across the region.
Frequently Asked Questions
How has infrastructure in Latin America improved medical investigation?
Over the past decade, infrastructure growth in Latin America has enhanced its medical investigation framework, establishing the region as an emerging hub for medical studies. Major cities, especially in Brazil, Mexico, and Argentina, now have cutting-edge facilities that meet international standards.
What types of research studies are being conducted in Latin America?
A diverse array of research studies is being conducted in Latin America, ranging from early-phase investigations to post-market assessments, facilitated by advanced technology and skilled professionals.
What legislative changes have been made in Brazil to support medical research?
Brazil has enacted Law 14.874/24, which streamlines the evaluation process for research studies, accelerating the initiation of investigations and reflecting a strong commitment to enhancing research capabilities.
Why is Colombia considered a premier destination for first-in-human clinical studies?
Colombia offers significant competitive advantages, including cost reductions exceeding 30% compared to North America and Western Europe, a quick IRB/EC and MoH review process of 90-120 days, and a highly ranked healthcare system.
What is the patient recruitment situation in Colombia?
Colombia has a population of over 50 million with 95% coverage under universal healthcare, leading to robust patient recruitment for clinical studies.
What financial incentives exist for investments in medical research in Colombia?
Investments in science, technology, and innovation projects in Colombia can benefit from a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and around $10 million in government grants.
How does bioaccess® contribute to clinical studies in Latin America?
Bioaccess® specializes in providing cost-effective, high-quality medical device CRO services, including regulatory approval, site activation, participant recruitment, and data management, facilitating expedited study outcomes.
What role does collaboration play in the growth of medical trials in Latin America?
Collaboration between public and private sectors has fostered an environment that promotes innovation and efficiency, leading to decentralized studies and the integration of digital technologies in medical research.
What certification process do hospitals in Colombia undergo for conducting trials?
Hospitals in Colombia must undergo a rigorous ICH/GCP certification process to conduct trials with pharmaceutical drugs, ensuring quality assurance in studies.
What are some benefits of conducting clinical studies in South America?
Benefits include cost-effectiveness, access to diverse patient populations, faster patient recruitment, and supportive regulatory environments that streamline study authorizations.

