Overview
The article addresses the essential regulatory considerations for conducting clinical trials in Argentina. It emphasizes the pivotal role of the National Administration of Medicines, Food and Medical Technology (ANMAT) and underscores the importance of adhering to ethical standards and international guidelines. The structured approval process is outlined, along with recent trends in research funding and participation. Additionally, it discusses challenges such as economic instability and logistical issues, all of which highlight the necessity for a robust regulatory framework to facilitate effective medical research in the country.
Introduction
In recent years, Argentina has emerged as a significant player in the global clinical trial landscape, driven by a robust regulatory framework and a commitment to ethical research practices. The National Administration of Medicines, Food and Medical Technology (ANMAT) plays a pivotal role in this ecosystem, ensuring that clinical trials adhere to stringent safety and efficacy standards.
As the country continues to refine its regulatory processes, stakeholders are increasingly drawn to Argentina's potential for conducting innovative medical research.
This article delves into the intricacies of the regulatory environment governing clinical trials in Argentina, highlighting key authorities, approval processes, and the challenges faced by researchers in this evolving landscape. Understanding these elements is essential for navigating the complexities of clinical trials and leveraging Argentina's capabilities effectively.
Explore the Regulatory Framework for Clinical Trials in Argentina
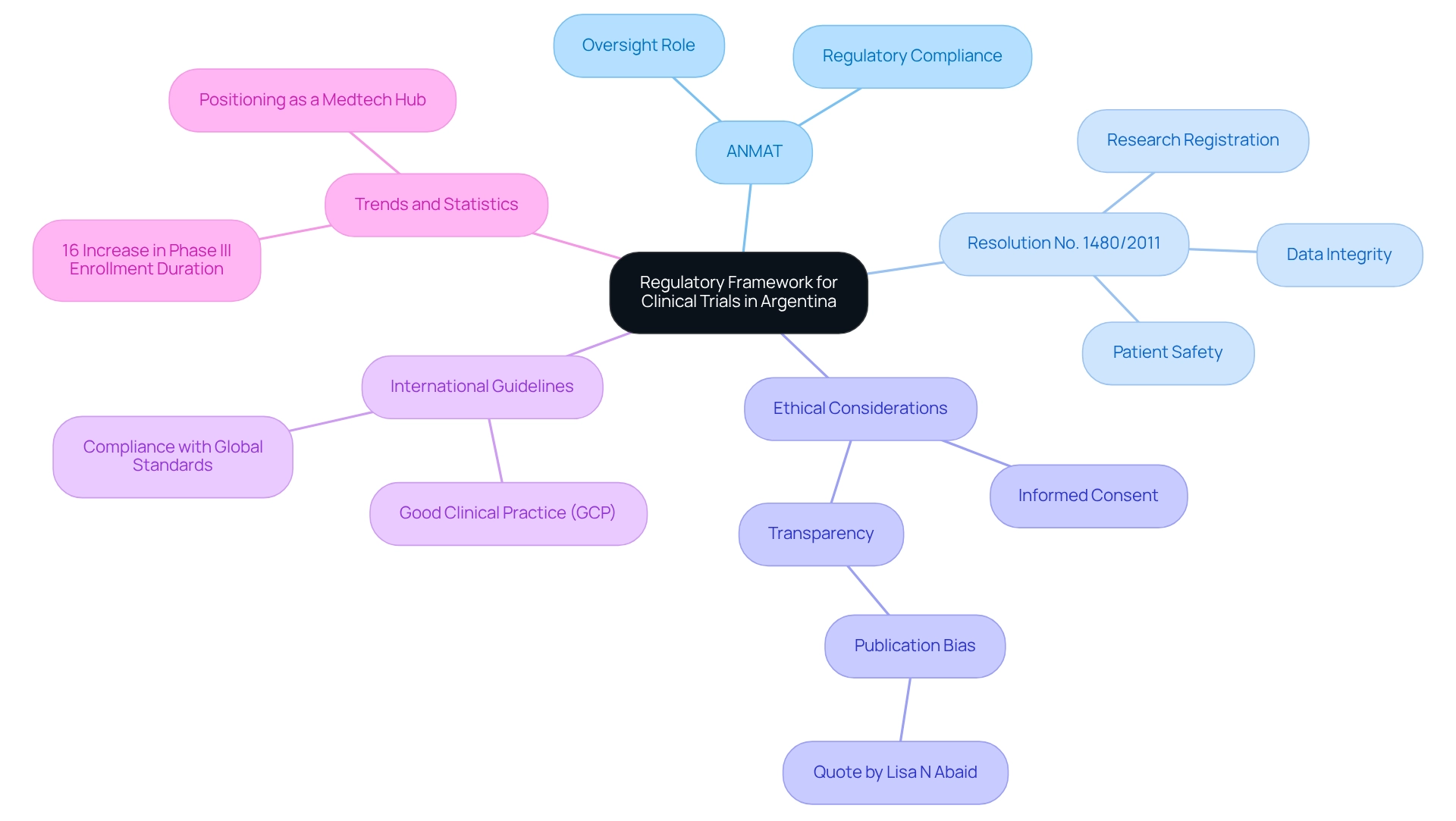
The regulatory framework for medical studies in Argentina encompasses vital considerations for trials, primarily overseen by the National Administration of Medicines, Food and Medical Technology (ANMAT). A pivotal element of this framework is Resolution No. 1480/2011, which delineates the essential criteria for conducting research involving human subjects, with a focus on ethical aspects, patient safety, and data integrity.
This resolution is indispensable for researchers and sponsors, as it provides a structured approach to navigating the regulatory landscape of trials in Argentina, alongside the complexities of approval and execution.
As we look toward 2025, the governance structure continues to evolve, reflecting Argentina's commitment to enhancing its research environment in healthcare. Adhering to international guidelines, particularly Good Clinical Practice (GCP), is crucial, ensuring that studies align with global standards for safety and efficacy. Notably, the integration of research study registration into the oversight framework is being promoted to bolster transparency and ethical standards.
As Lisa N Abaid observed, "Publication bias is the selective publishing of favorable or statistically significant results," underscoring the pressing need for transparency in medical research.
Recent statistics reveal a 16% increase in the duration of Phase III research enrollment, underscoring the necessity for robust oversight processes. This trend accentuates the importance of an effective regulatory environment, including regulatory considerations for trials in Argentina, in facilitating timely execution of studies and attracting medtech firms.
At bioaccess®, we are committed to ensuring information security and client confidence throughout the research process. Our grievance and data protection protocols are meticulously designed to address client concerns with compliance and transparency. Should you have any queries or concerns regarding the processing of your information, please reach out to our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
With over 20 years of experience in overseeing health-related studies, bioaccess® delivers comprehensive services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Health Follow-Up Studies. Understanding the regulatory considerations for trials in Argentina and other compliance nuances is crucial for stakeholders aiming to effectively leverage the country's research capabilities. As the nation continues to enhance its oversight structure, staying informed about changes and compliance obligations will be vital for the successful execution of studies.
Identify Key Regulatory Authorities and Their Roles
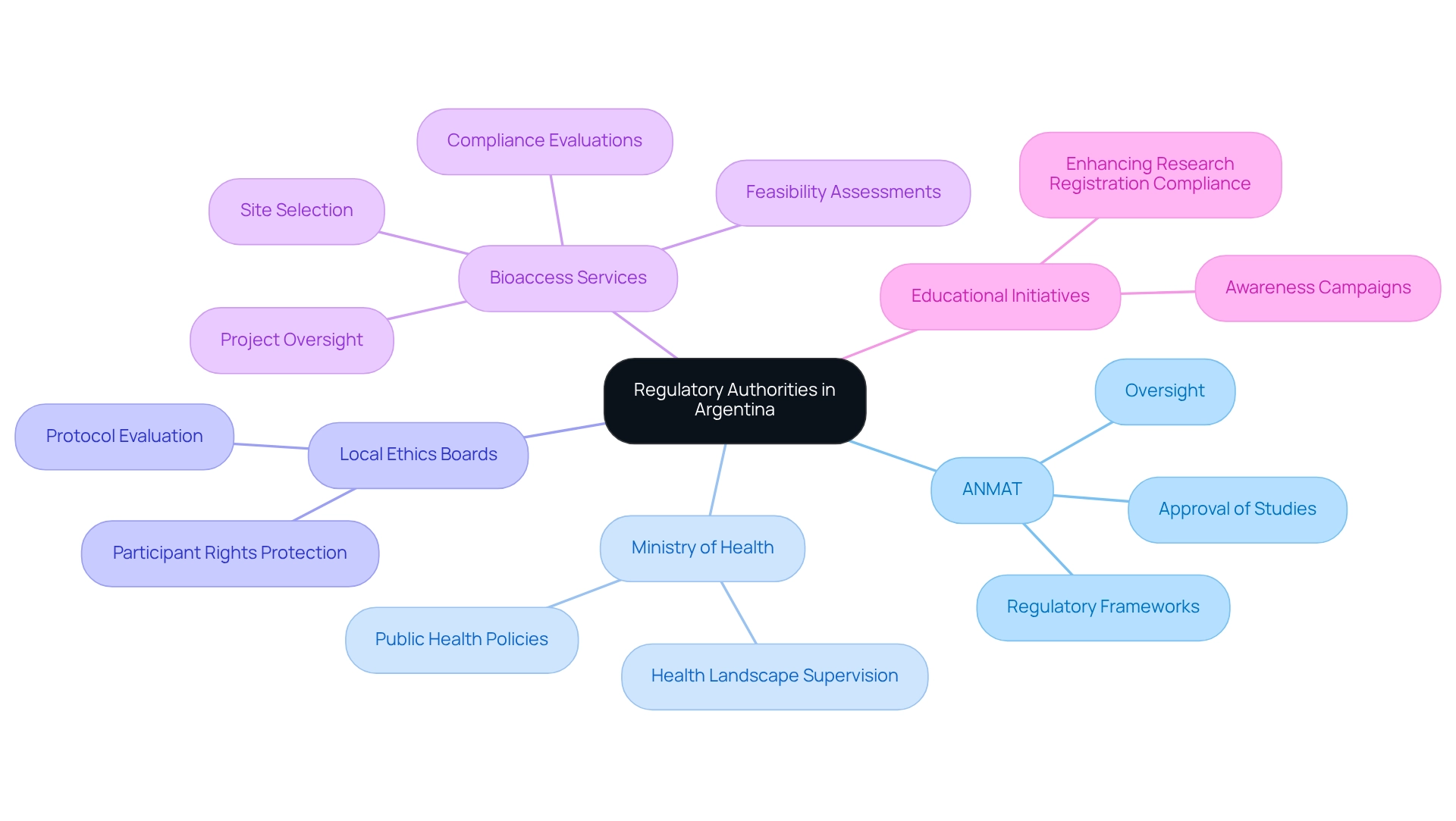
The National Administration of Drugs, Food and Medical Technology (ANMAT) serves as the principal regulatory authority overseeing research studies, encompassing crucial regulatory considerations for trials in Argentina. ANMAT holds the responsibility for the approval and oversight of these studies, necessitating a comprehensive understanding of regulatory frameworks to ensure adherence to both national and international standards. Complementing ANMAT's pivotal role is the Ministry of Health, which formulates public health policies and supervises the broader health landscape.
Local ethics boards are integral to this process, meticulously evaluating study protocols to uphold ethical standards and protect participant rights. The collaboration among these organizations is vital for a streamlined approval process. For instance, recent findings indicate that educational initiatives aimed at enhancing research registration compliance could significantly elevate awareness of obligations among sponsors and researchers. Such initiatives are imperative, particularly given that 26.9% of participants in a recent study reported that scientific journals mandate proof of registration for publication.
Moreover, supporting local investigator-sponsor-initiated studies is essential for fostering a robust research environment in Argentina. Additionally, bioaccess offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These services not only simplify the compliance process but also stimulate the local economy by creating jobs and improving healthcare outcomes. The governance landscape in the country is evolving, with ANMAT actively engaging in discussions regarding regulatory considerations for trials in Argentina, emphasizing the importance of research registration and the challenges faced by sponsors and researchers.
This structured approach not only fosters transparency but also aligns with global standards, thereby enhancing the credibility of medical research conducted in the region. As of 2025, the roles of ANMAT and the Ministry of Health remain critical in shaping the regulatory landscape for trials in Argentina. Their collaborative efforts, in conjunction with local ethics committees and the services provided by bioaccess, are essential for upholding high standards of research integrity and participant safety, ultimately advancing global health through international collaboration and innovation in Medtech.
Navigate the Approval Process and Required Documentation
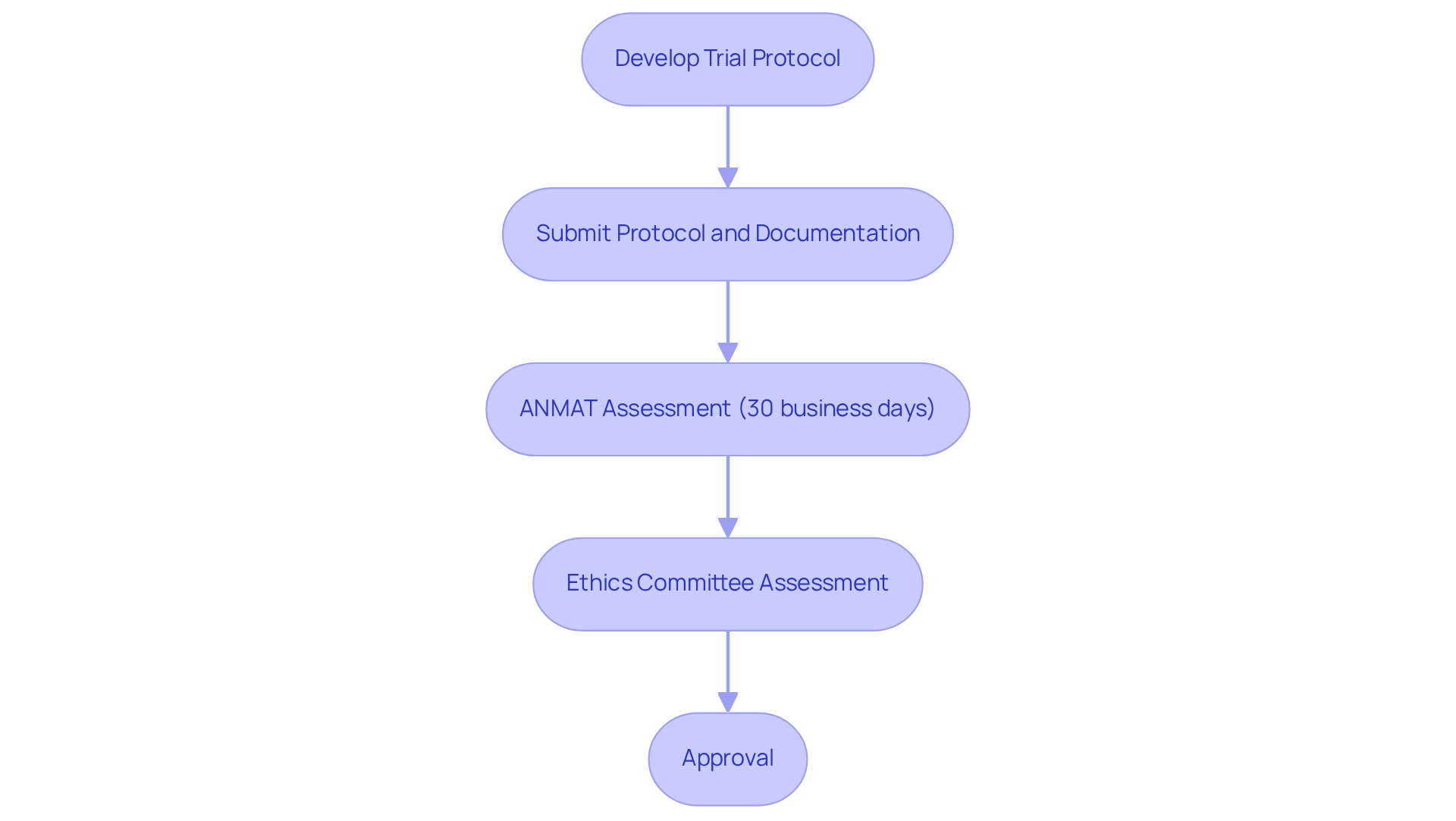
The clinical study approval process in Argentina encompasses multiple steps and necessitates meticulous preparation, considering the regulatory landscape for trials in the country. Researchers must initially develop a comprehensive trial protocol that articulates the study's objectives, methodology, and ethical considerations. For example, a protocol may delineate specific endpoints for a new medical device designed to enhance patient outcomes.
This protocol is submitted to ANMAT, accompanied by essential documentation such as informed consent forms, investigator qualifications, and safety monitoring plans. ANMAT typically concludes its assessment within 30 business days, underscoring the nation's commitment to effective oversight practices.
In parallel with ANMAT's evaluation, local ethics committees are mandated to assess and endorse the protocol, ensuring adherence to ethical standards. This dual-layered approval process is vital for minimizing delays and ensuring compliance with regulatory considerations for trials in Argentina. Notably, the country boasts low drop-out rates and high enrollment and compliance rates, which contribute to the generation of quality data for research purposes.
The robust enrollment and adherence rates are particularly advantageous, as they enhance the reliability of the information collected during studies.
Recent trends indicate a significant increase in funding for research within the Andean Region, escalating from $3-4 million to over $50 million annually. This surge highlights the growing interest in conducting research in countries such as Peru and Colombia. Furthermore, the duration of Phase III research study enrollment has increased by 16%, reflecting the evolving landscape of studies in the area.
As the population ages, with 30% under 14 years old, the potential market for new medications is expanding, further emphasizing the regulatory considerations for trials in Argentina and the significance of an efficient approval process for research studies in the country. As Christof Baron, CEO, remarked, 'Statista is an excellent source of information, and quite useful for handling daily tasks,' highlighting the importance of reliable data sources in navigating the complexities of medical studies. Additionally, the average consent duration for research studies in the nation in 2025 is expected to remain competitive, reinforcing the country's role as a favorable site for medical research.
At bioaccess®, we leverage our expertise in managing research studies to navigate these compliance pathways effectively, ensuring adherence and promoting successful outcomes for our clients. Our extensive research management services encompass Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). By concentrating on these specific types of studies, we not only enhance the efficiency of the approval process but also contribute to local economies through job creation and improved healthcare outcomes, establishing ourselves as a leading contract research organization in Latin America.
Address Challenges and Considerations in the Argentine Context
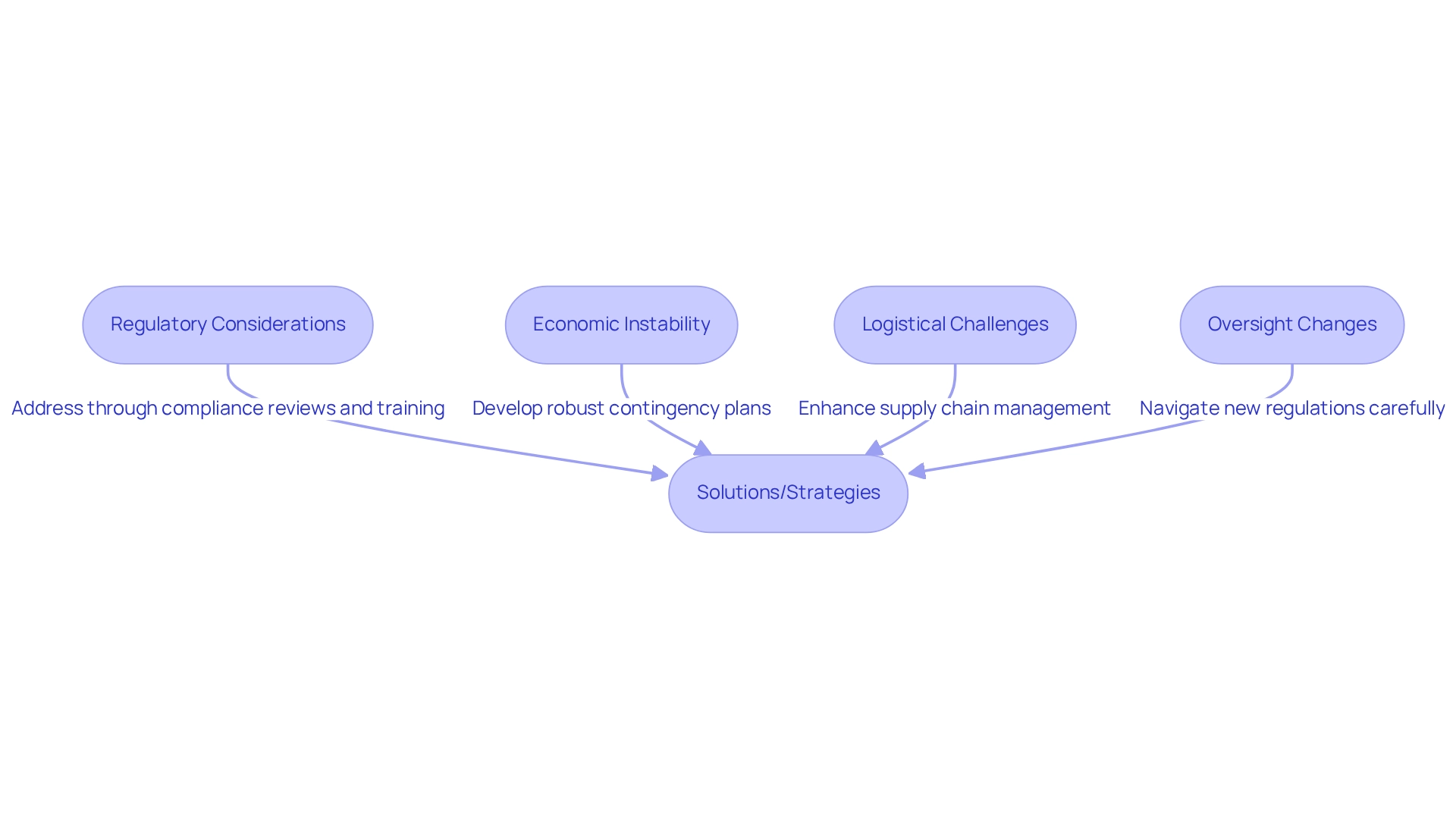
Conducting medical studies in Argentina presents several challenges, primarily stemming from regulatory considerations for trials in the country, economic instability, and logistical complications. The nation has been grappling with high inflation rates, which complicate budgeting and financing for clinical studies. Additionally, currency controls exacerbate these issues, rendering financial planning a formidable task for researchers.
The oversight environment is also undergoing significant changes. Recent reforms aim to streamline the approval process; however, they introduce new regulatory considerations for trials in Argentina that researchers must navigate with care. While these changes are designed to facilitate quicker access to the initiation of experiments, they may lead to inconsistencies in regulatory interpretations, particularly concerning regulatory considerations for trials in Argentina, which can impact timelines and resource allocation.
Logistical challenges, such as supply chain interruptions for research materials, further complicate the landscape. These obstacles can delay proceedings and affect the quality of data collected. A study on medical research reporting underscores the importance of adhering to rigorous protocols to ensure transparency and reliability, highlighting the need for meticulous planning when faced with these challenges.
Despite these hurdles, bioaccess offers comprehensive clinical study management services aimed at mitigating these issues. Their expertise encompasses feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting. These services not only enhance the likelihood of successful experimentation but also bolster the local economy by creating jobs and improving healthcare outcomes.
Researchers who successfully navigate the economic instability in Argentine studies underscore the significance of regulatory considerations for trials in Argentina by developing robust contingency plans and fostering strong relationships with local stakeholders. Expert opinions emphasize the necessity of training and education to elevate the quality of randomized research studies, addressing the challenges that may arise in this complex environment. As Julio G. Martinez-Clark, CEO, noted, "The government of Colombia appears to be the sole nation in Latin America with a proactive effort to draw more research studies as part of its strategy to evolve into a knowledge economy by 2031."
This observation highlights the competitive landscape for medical studies in the region. As Argentina continues to develop as a clinical trial destination, comprehending these dynamics will be essential for researchers aiming to conduct effective studies in the area.
Conclusion
Argentina's emergence as a pivotal player in the global clinical trial landscape is highlighted by its robust regulatory framework, particularly through the initiatives of the National Administration of Medicines, Food and Medical Technology (ANMAT). The structured approach established by Resolution No. 1480/2011 guarantees that ethical considerations, patient safety, and data integrity are prioritized in clinical research. As the regulatory landscape evolves, adherence to international standards such as Good Clinical Practice (GCP) becomes essential, fostering an environment that is conducive to innovative medical research.
The collaborative efforts of ANMAT, the Ministry of Health, and local ethics committees are vital in navigating the complexities of the clinical trial approval process. With a strong emphasis on transparency and adherence to ethical standards, these entities work in unison to enhance the credibility of clinical research in Argentina. Furthermore, the support provided by organizations like bioaccess, which delivers comprehensive clinical trial management services, plays a crucial role in streamlining processes and addressing the unique challenges faced by researchers.
Despite ongoing challenges such as economic instability and logistical complexities, the continuous reforms and investments in the clinical trial sector indicate a positive trajectory for Argentina's research capabilities. By comprehensively understanding the intricacies of the regulatory environment and leveraging local expertise, stakeholders can effectively navigate this landscape and capitalize on Argentina's potential as a prime location for clinical trials. As the country continues to refine its processes and embrace international standards, it is poised to emerge as a leading hub for innovative medical research in the region.
Frequently Asked Questions
What regulatory body oversees medical studies in Argentina?
The National Administration of Medicines, Food and Medical Technology (ANMAT) oversees medical studies in Argentina.
What is Resolution No. 1480/2011 and why is it important?
Resolution No. 1480/2011 outlines essential criteria for conducting research involving human subjects, focusing on ethical aspects, patient safety, and data integrity. It is crucial for researchers and sponsors to navigate the regulatory landscape of trials in Argentina.
How is Argentina's research governance expected to evolve by 2025?
Argentina's research governance is expected to evolve to enhance its healthcare research environment, reflecting a commitment to international guidelines, particularly Good Clinical Practice (GCP), and promoting research study registration for improved transparency and ethical standards.
What is publication bias and why is it significant in medical research?
Publication bias refers to the selective publishing of favorable or statistically significant results. It is significant because it highlights the need for transparency in medical research to ensure that all results are reported, contributing to the integrity of scientific knowledge.
What recent trend has been observed in Phase III research enrollment?
There has been a 16% increase in the duration of Phase III research enrollment, indicating a necessity for robust oversight processes in the regulatory environment to facilitate timely execution of studies.
What services does bioaccess® provide related to health-related studies?
Bioaccess® provides a range of services including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Health Follow-Up Studies.
How does bioaccess® ensure client confidence and information security during research?
Bioaccess® has grievance and data protection protocols designed to address client concerns regarding compliance and transparency throughout the research process.
Who should be contacted for queries regarding information processing at bioaccess®?
Queries regarding information processing can be directed to the Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com.




