Overview
The article centers on the requirements established by ANMAT for conducting medtech clinical trials in Argentina, underscoring the critical nature of compliance with regulatory standards to ensure the safety and efficacy of medical products. It delineates essential requirements, including:
- Submission of a Clinical Trial Application
- Obtaining ethics committee approval
- Adhering to Good Clinical Practice
- Maintaining transparency throughout the research process
These measures are pivotal for fostering trust and advancing medical innovation in the region, illustrating the indispensable role of regulatory adherence in the clinical research landscape.
Introduction
In the realm of medical innovation, navigating regulatory landscapes is paramount for success, particularly in Argentina, where the National Administration of Medicines, Food and Medical Technology (ANMAT) plays a critical role. As the regulatory authority tasked with ensuring the safety and efficacy of medical products, ANMAT's influence extends to the approval and oversight of clinical trials. Therefore, it is essential for researchers and Medtech companies to understand its frameworks.
Recent developments aimed at streamlining approval processes and enhancing transparency indicate that the regulatory environment is evolving, presenting both challenges and opportunities for stakeholders. This article delves into the intricacies of ANMAT's regulations, highlighting key requirements for clinical trials, the importance of compliance, and best practices for navigating this complex landscape.
Ultimately, it positions Argentina as a burgeoning hub for medical technology innovation.
Overview of ANMAT: The Regulatory Authority in Argentina
The National Administration of Medicines, Food and Medical Technology (ANMAT) stands as Argentina's regulatory authority, tasked with ensuring the safety, efficacy, and quality of medical products, including medical devices and pharmaceuticals. Established under the Ministry of Health, ANMAT plays a pivotal role in upholding both national and international standards, which are essential for the approval and supervision of research studies.
In 2024, ANMAT authorized a significant number of research studies, underscoring its commitment to advancing medical innovation while safeguarding public health. The agency's primary responsibilities encompass:
- Evaluating research study applications
- Conducting thorough inspections
- Enforcing regulations that protect the welfare of the population
Beginning in 2025, revisions to Argentina's medical device regulations have streamlined the approval process, enhancing efficiency for businesses, including those collaborating with bioaccess®, in navigating the complexities of research.
ANMAT's oversight is characterized by a proactive approach to monitoring research studies, ensuring compliance with established guidelines. This includes the implementation of recent changes aimed at increasing transparency and efficiency in the approval process. Successful research studies conducted under these regulatory frameworks demonstrate ANMAT's effectiveness in fostering a robust environment for medical investigation, particularly for innovative medical devices developed by Medtech startups.
Expert opinions highlight the critical role of ANMAT in research studies within Argentina, emphasizing its importance in bridging innovative medical technologies with regulatory compliance. The agency's continuous efforts to adapt to the evolving needs of the industry position it as a key player in the advancement of medical devices and pharmaceuticals in the region. This is further supported by bioaccess®'s expertise in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Bioaccess® is dedicated to providing affordable, high-quality research services, collaborating closely with Medtech startups to expedite study outcomes.
Understanding the structure and functions of Argentina's health authority is essential for any organization intending to conduct research studies in the country, as it directly influences ANMAT's requirements for medtech clinical trials and the approval process, along with compliance obligations. Case studies of successful clinical trials under the agency's oversight illustrate its capability to support the growth of the Medtech sector while ensuring the highest standards of safety and efficacy.
In a broader context, statistics on FDA first premarket approvals for medtech products from 2005 to 2022 reveal a growing trend in approvals, serving as a comparative backdrop for ANMAT's activities. Additionally, the E22 Guideline, approved by the ICH Assembly in June 2023, aims to enhance the incorporation of patient preference information in pharmaceutical product development, as noted by Ms. Robyn Bent, highlighting the global standards that influence local governance frameworks. Furthermore, the case study titled "Data Exclusivity for New Medicines in Colombia" illustrates the regulatory dynamics that can affect research approvals and market access, providing a real-world example that enriches the discussion on the regulatory framework.
Recent statistics regarding ANMAT's role in research approvals in Argentina further elucidate its impact and effectiveness within the Medtech sector.
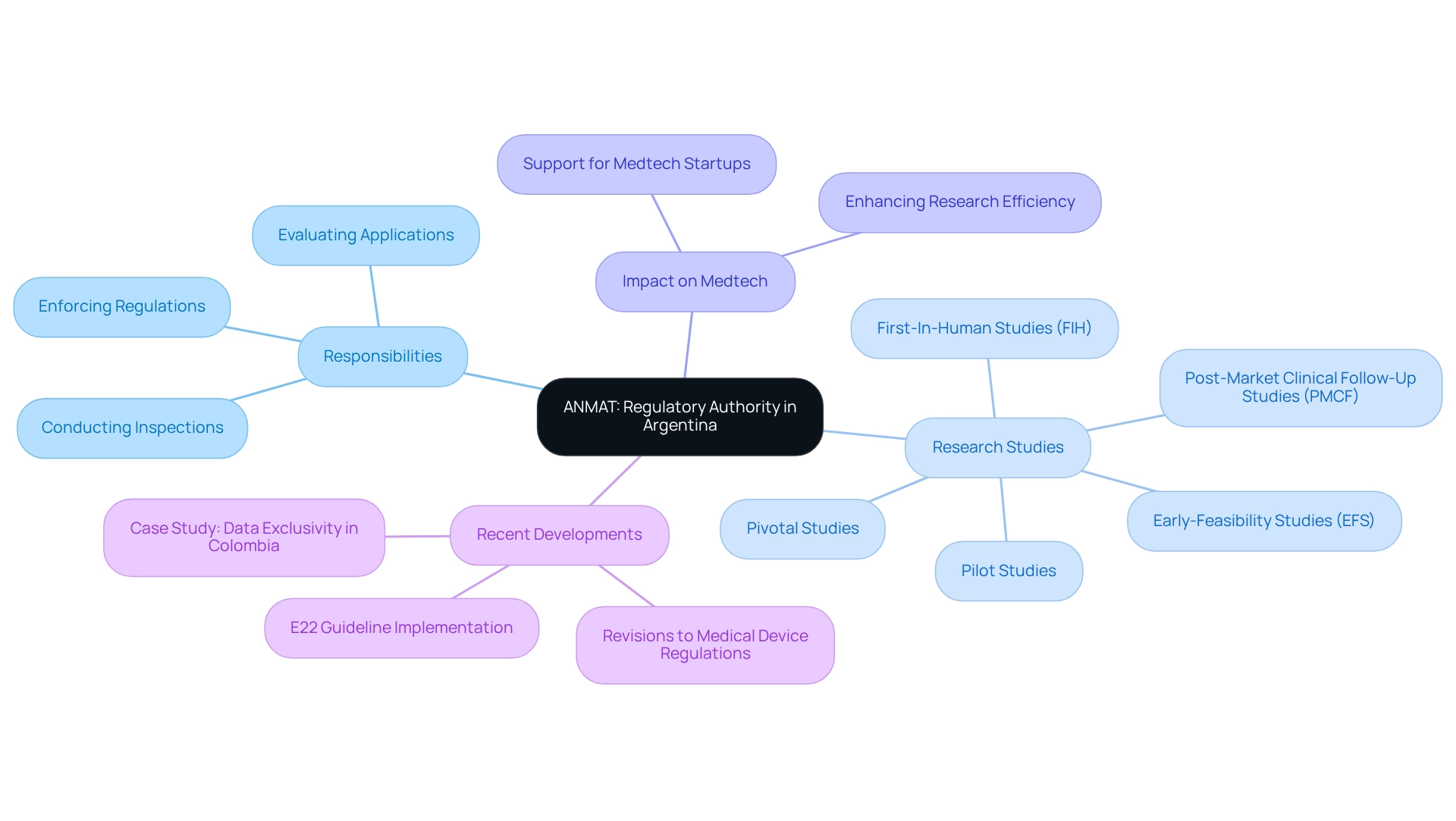
Key ANMAT Requirements for Clinical Trials in Medtech
Carrying out clinical studies in Argentina necessitates compliance with the ANMAT requirements for medtech clinical trials, which are crucial for ensuring adherence and facilitating effective study implementation. These requirements include:
- Submission of a Clinical Trial Application (CTA): Researchers must prepare and submit a comprehensive CTA that encompasses the study protocol, informed consent forms, and qualifications of the investigators involved. This submission acts as the basis for the approval process of the examination.
- Ethics Committee Approval: Before ANMAT can review the CTA, it is imperative that the study receives approval from an accredited ethics committee. This step is crucial to ensure that the experiment meets ethical standards and protects the rights and welfare of participants. Recent statistics indicate that approximately 85% of clinical studies in Argentina successfully obtain ethics committee approval, reflecting a robust framework for ethical oversight in clinical research.
- Good Clinical Practice (GCP) Compliance: All studies must adhere to GCP guidelines, designed to uphold the integrity of data and safeguard participant rights. Compliance with these guidelines is not only a regulatory requirement but also a best practice that enhances the credibility of the research findings.
- Safety Monitoring: Researchers are required to enforce stringent safety monitoring protocols during the study. This includes the timely reporting of any adverse events, ensuring that participant safety remains a top priority during the study.
- Documentation and Record Keeping: Maintaining comprehensive documentation is vital for the integrity of the examination. This encompasses thorough documentation of participant information and data handling methods, which are essential for compliance with regulations and upcoming assessments.
Grasping the ANMAT requirements for medtech clinical trials is essential for the effective commencement and implementation of studies in the Medtech field. As of 2025, staying informed about any updates or changes to the ANMAT requirements for medtech clinical trials will further improve the efficiency and effectiveness of the research process. Furthermore, it is crucial to acknowledge that oversight reliance pathways in LATAM are not consistently adopted, which may influence the efficiency of drug approval processes in the region.
The necessity for clear data visibility in the Americas highlights the significance of efficient oversight procedures and accountability in research studies. As Urimara Argotti-Rodríguez noted, collaboration among stakeholders is essential for navigating these complexities. Furthermore, understanding the test data exclusivity granted by Colombia can provide a comparative perspective on regulatory practices in the region, relevant for Medtech companies operating across Latin America.
With bioaccess® at the forefront of research management services—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—companies can navigate these challenges effectively and capitalize on the opportunities within the Latin American Medtech landscape. Engage with bioaccess® to utilize our knowledge and guarantee the success of your research studies.
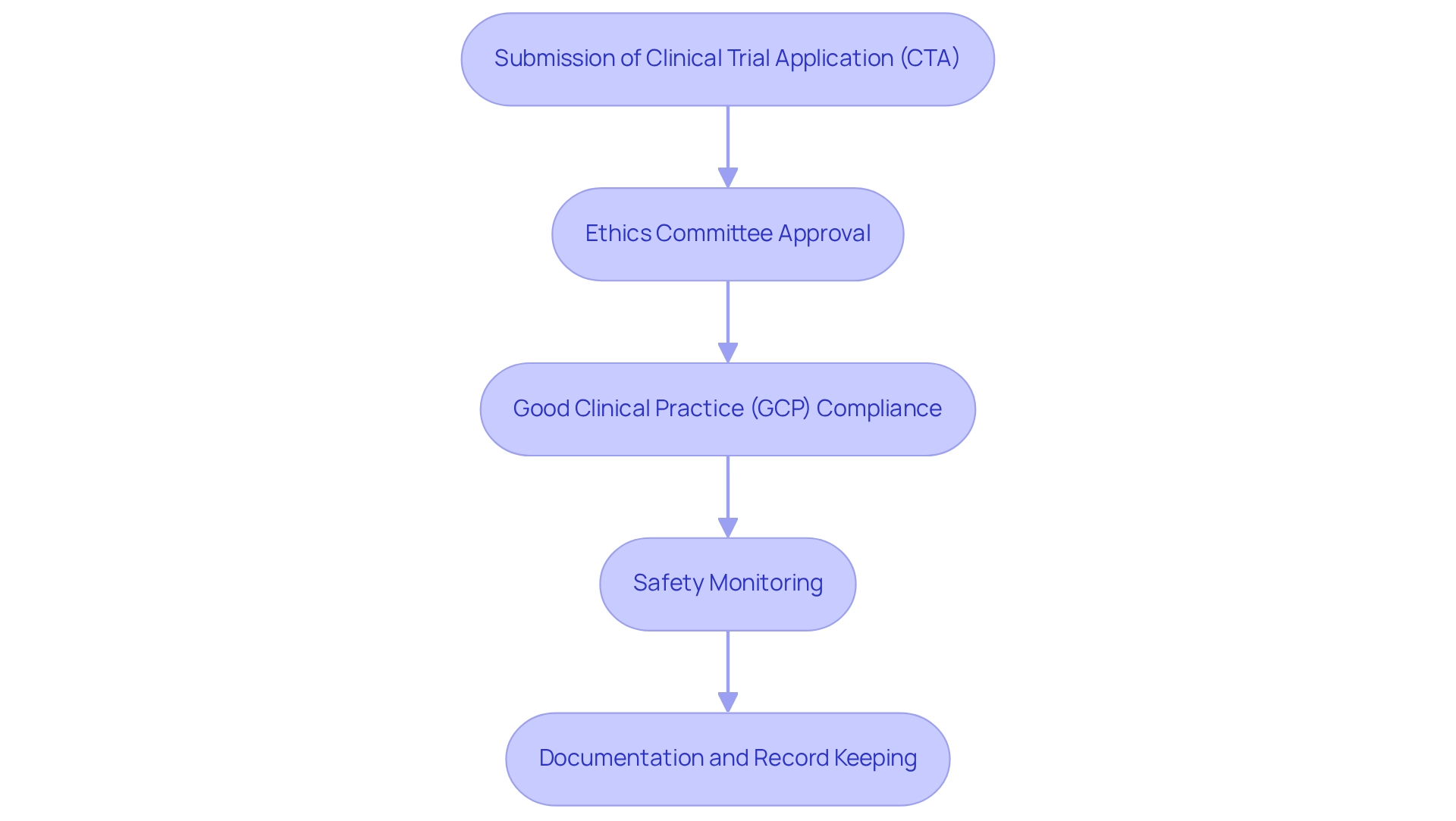
The Role of Transparency in ANMAT Regulations
Openness in medical studies serves as a cornerstone of the ANMAT requirements for medtech clinical trials, underscoring a commitment to ethical research practices. Key components of this framework include:
- Mandatory Registration: All clinical studies must be registered in a publicly accessible database. This initiative ensures that information about ongoing studies is readily available, fostering public trust and engagement in the research process.
- Disclosure of Results: Researchers are required to report the outcomes of their studies, encompassing both positive and negative findings. This requirement promotes accountability and enables informed decision-making among stakeholders, including healthcare professionals and patients.
- Informed Consent: Participants have the right to detailed information regarding the study's objectives, procedures, potential risks, and benefits. This transparency empowers individuals to make informed decisions about their participation, thereby enhancing ethical standards in research.
- Regular Reporting: ANMAT mandates that researchers deliver regular updates on study progress and any adverse events. This ongoing communication not only keeps stakeholders informed but also reinforces the integrity of the research process.
These transparency measures significantly bolster the credibility of medical research while adhering to ANMAT requirements for medtech clinical trials and safeguarding the rights and welfare of participants. In 2023, statistics from the Country Health Profiles indicated that approximately 75% of medical trials in Argentina complied with mandatory registration, reflecting a growing commitment to transparency in the research landscape. Furthermore, expert opinions emphasize that adherence to these regulations is essential for fostering trust in medical research.
Dr. Alanoud M. Almuhareb from the SFDA in Saudi Arabia stated, "This guideline is intended to complement and expand on ICH E11(R1) to provide a more comprehensive framework for using pediatric extrapolation in optimizing pediatric drug development," highlighting the significance of clarity in oversight frameworks. Additionally, media coverage by Clinical Leader has spotlighted research studies in Latin America and Colombia, illustrating the increasing awareness and scrutiny of these processes. Drawing parallels with Colombia's pricing regulation for medicines, which allows for free pricing but includes mechanisms for price control, illustrates the varying approaches to oversight transparency in Latin America.
Ultimately, these efforts lead to improved health outcomes and advancements in medical technology.
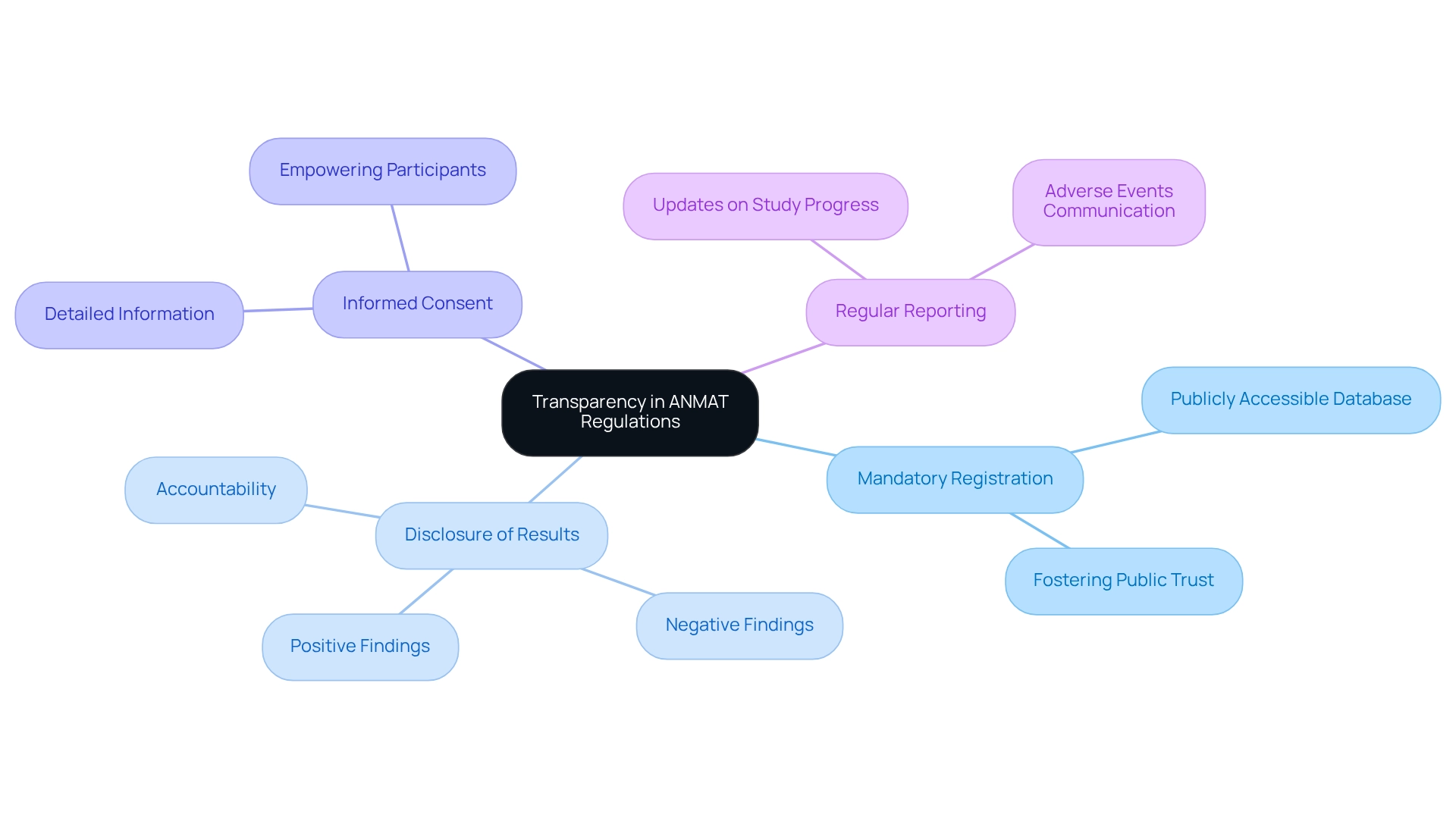
Challenges in Complying with ANMAT Requirements
Scientists maneuvering through the approval procedure frequently encounter numerous obstacles that can significantly influence their study timelines and overall success. Key issues include:
- Complex Documentation: The extensive and intricate documentation required for Clinical Trial Applications (CTAs) can be daunting, particularly for smaller organizations and startups. Many Medtech startups have reported that the volume and specificity of required documents often exceed their initial expectations, leading to delays in submission. As Ms. Robyn Bent observed, the E22 Guideline seeks to enhance the utilization of patient preference information as a component in pharmaceutical product development, emphasizing the significance of comprehensive documentation in compliance processes.
- Lengthy Approval Processes: The average time for ANMAT to approve clinical trial applications can extend beyond several months, which can stall trial initiation and disrupt project timelines. Recent reports indicate that delays in approval processes have become increasingly common, with some applications taking up to six months or longer for review. This situation reflects the challenges addressed at the RAPS Euro Convergence conference, where industry experts convened to tackle compliance issues and remain informed about the changing environment.
- Policy Changes: The oversight landscape is continually evolving, with frequent updates that can create uncertainty for researchers. Staying abreast of these changes is crucial, as failure to adapt protocols in line with new regulations can lead to compliance issues and further delays. For instance, the implementation of new guidelines, such as those adopted by EDA, Egypt on 28 October 2024, may impact regulatory processes and necessitate adjustments in compliance strategies.
- Resource Limitations: Smaller organizations often struggle with limited resources and expertise, making it challenging to navigate the complex compliance environment effectively. This lack of capacity can lead to incomplete submissions or misunderstandings of regulatory requirements, ultimately hindering compliance. The establishment of the E14/S7B Implementation Working Group in November 2018 underscores ongoing efforts to develop guidelines that could assist researchers in overcoming these challenges.
- Cultural and Language Barriers: For international sponsors, understanding local regulations and cultural nuances presents additional hurdles. Miscommunication or misunderstanding of local practices can complicate the approval process, emphasizing the need for local expertise.
Addressing these challenges necessitates meticulous planning, adequate resource allocation, and a comprehensive understanding of the regulatory framework. By fostering collaboration and leveraging local knowledge, researchers can enhance their chances of successful compliance with ANMAT requirements. bioaccess® is prepared to assist in this journey, providing extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, ensuring a streamlined process for medical device studies in Latin America.
Recent Developments in Latin American Clinical Trial Regulations
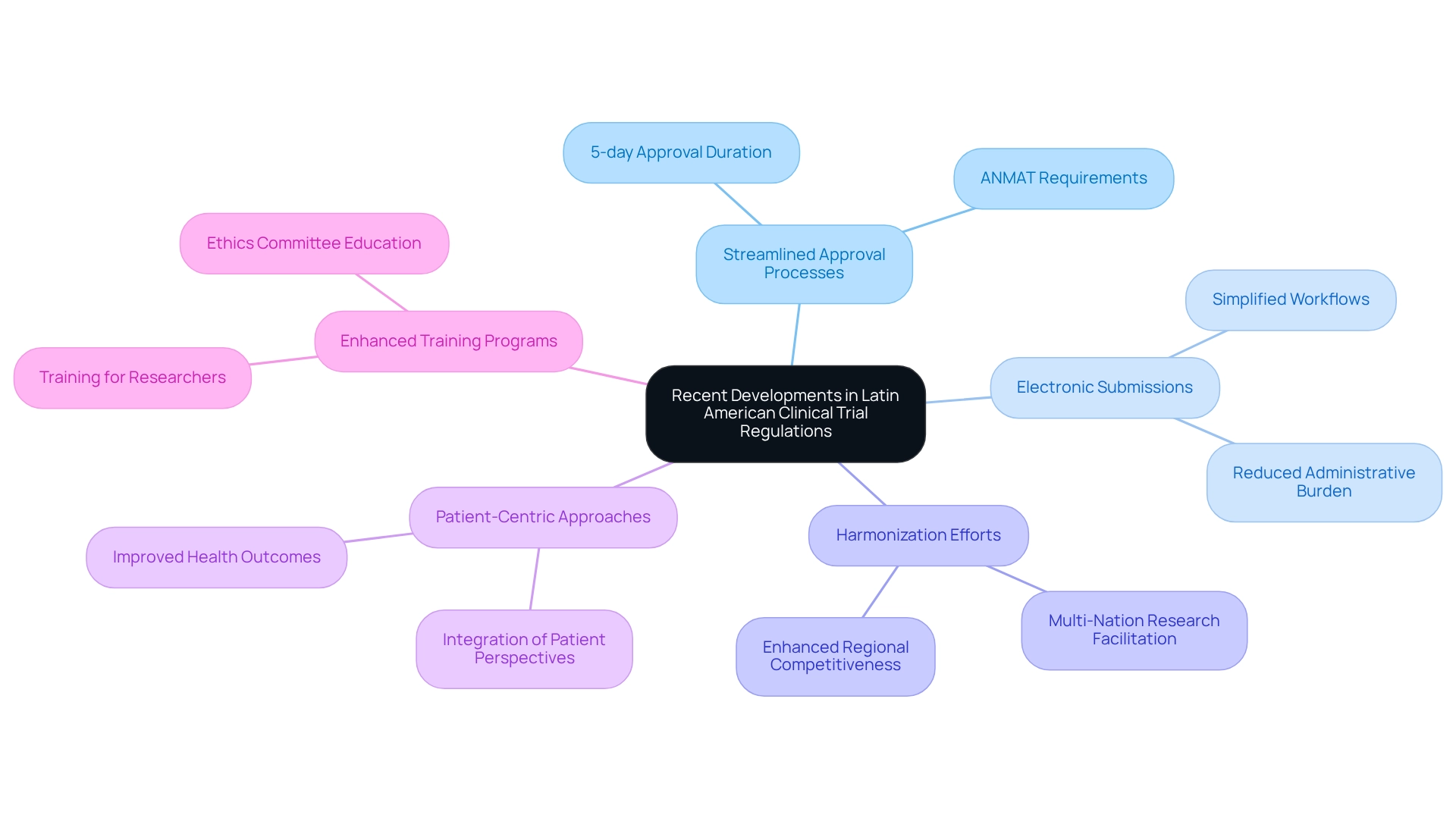
Recent changes in research regulations across Latin America, particularly in Argentina, have significantly transformed the landscape for medical studies. Key advancements include:
- Streamlined Approval Processes: The regulatory agency has implemented measures designed to accelerate the approval timeline for trials involving human subjects while adhering to ANMAT requirements for medtech clinical trials. This initiative has markedly improved Argentina's attractiveness as a location for medical research, with median approval durations noted at around 5 days. This demonstrates a commitment to efficiency in oversight procedures. According to Evandro de Azambuja from the Clinical Trials Support Unit at the Jules Bordet Institute, 'The ANMAT requirements for medtech clinical trials streamline approval processes that are essential for promoting innovation and drawing international research to Argentina.'
- Introduction of Electronic Submissions: The introduction of electronic submission platforms has revolutionized the application process, allowing researchers to manage compliance requirements with greater ease and speed. This shift not only simplifies workflows but also reduces the administrative burden on study sponsors.
- Harmonization Efforts: Collaborative initiatives among Latin American nations are underway to harmonize regulatory frameworks. This effort aims to facilitate multi-nation research studies, thereby enhancing regional competitiveness and promoting a more unified approach to medical research across borders.
- Focus on Patient-Centric Approaches: Recent regulations underscore the importance of patient involvement in clinical studies. By integrating patient perspectives into trial design and execution, researchers can elevate the relevance and impact of their studies, ultimately leading to improved health outcomes.
- Enhanced Training Programs: ANMAT has introduced extensive training initiatives for researchers and ethics committees, aimed at deepening their understanding of the ANMAT requirements for medtech clinical trials and best practices. This initiative is vital for ensuring that all stakeholders are well-equipped to navigate the evolving regulatory landscape.
Furthermore, bioaccess® has played a pivotal role in establishing Barranquilla, Colombia, as a prominent location for research studies through its partnership with Caribbean Health Group. This collaboration seeks to attract more research projects to the region, supported by Colombia's Minister of Health, thus contributing to local economic development and job opportunities. Additionally, a case study analyzing transparency in pharmaceutical data across Argentina, Brazil, and Colombia reveals significant limitations in data sharing and public policy decision-making.
These challenges can impact the regulatory landscape and the overall effectiveness of trials in Argentina.
These advancements reflect a strong commitment to enhancing the research environment in Argentina, positioning the country as a crucial center for Medtech innovation in the region.
Best Practices for Navigating ANMAT Requirements
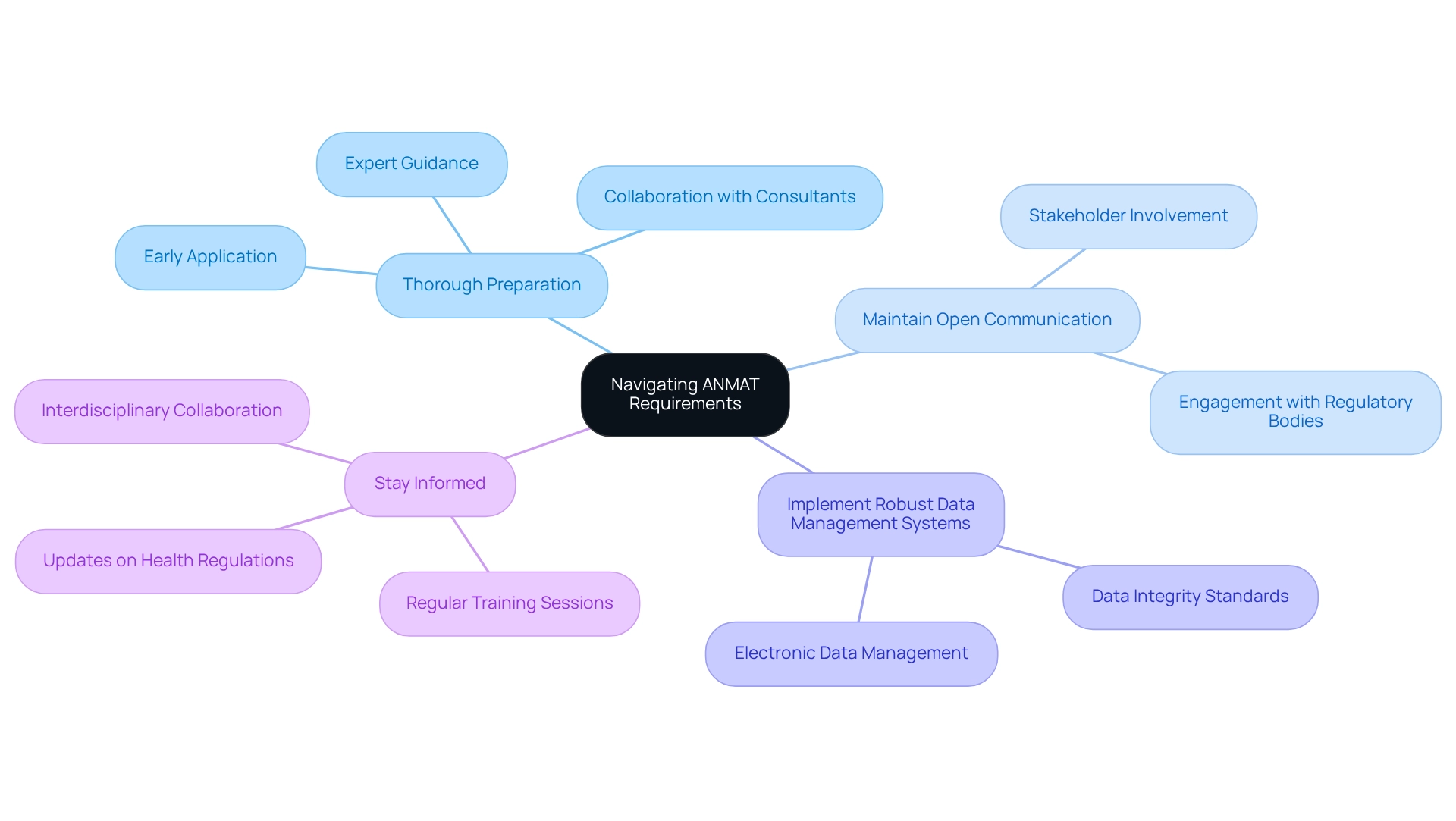
To effectively navigate regulatory requirements, researchers should adopt the following best practices:
- Thorough Preparation: Initiate the application process well in advance. Ensuring that all necessary documentation is meticulously prepared and accurate is crucial to prevent any potential delays in approval. bioaccess® provides extensive clinical trial management services, such as Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, that can aid in this preparation, ensuring that all documentation complies with the strict standards set by the regulatory authority. Collaborating with local compliance consultants or legal specialists who possess a deep understanding of the ANMAT requirements for medtech clinical trials can significantly streamline the application process. Their expertise offers invaluable guidance tailored to the specific nuances of the legal framework in Argentina. The effectiveness of local advisory consultants in navigating these requirements has been underscored by various studies, highlighting their role in facilitating smoother application processes and enhancing overall compliance rates in Argentina. bioaccess®'s team includes experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and can provide critical insights.
- Maintain Open Communication: Establishing clear and consistent lines of communication with regulatory bodies and ethics committees is essential. This proactive approach allows for the prompt resolution of any questions or concerns that may arise during the application process. bioaccess® emphasizes the importance of communication in their project management approach, ensuring that all stakeholders are informed and engaged.
- Implement Robust Data Management Systems: Utilizing electronic data management systems can enhance the efficiency of documentation processes. These systems not only simplify record management but also guarantee adherence to regulatory bodies' strict standards for data integrity and accessibility. bioaccess® employs advanced data management solutions to support their clinical trial services, ensuring that all data is managed effectively.
- Stay Informed: Regularly reviewing updates to health regulations is vital. Engaging in training sessions and workshops can assist researchers in staying updated on best practices and compliance tactics, thus improving their readiness for any policy changes. The CITI Program, accredited by the International Accreditors for Continuing Education and Training (IACET), illustrates the significance of continuous education in upholding adherence to regulatory standards. bioaccess® also offers training and resources to keep researchers updated on the latest compliance developments. Furthermore, the formation of the E14/S7B Implementation Working Group highlights the necessity for standardized methods in oversight processes, which can improve comprehension of risk assessment strategies pertinent to the organization. As Dr. Lisa V. Adams observes, teamwork and interdisciplinary methods are essential in managing compliance requirements. Moreover, remaining updated on advancements in the Medtech sector, like Niowave's application of a superconducting electron linear accelerator for isotope generation, can offer perspectives that might influence regulatory methods. By incorporating these practices, researchers can greatly enhance their likelihood of conducting successful studies while ensuring compliance with the ANMAT requirements for medtech clinical trials, aided by bioaccess®'s proficiency in study management.
Implications of ANMAT Regulations for the Medtech Industry
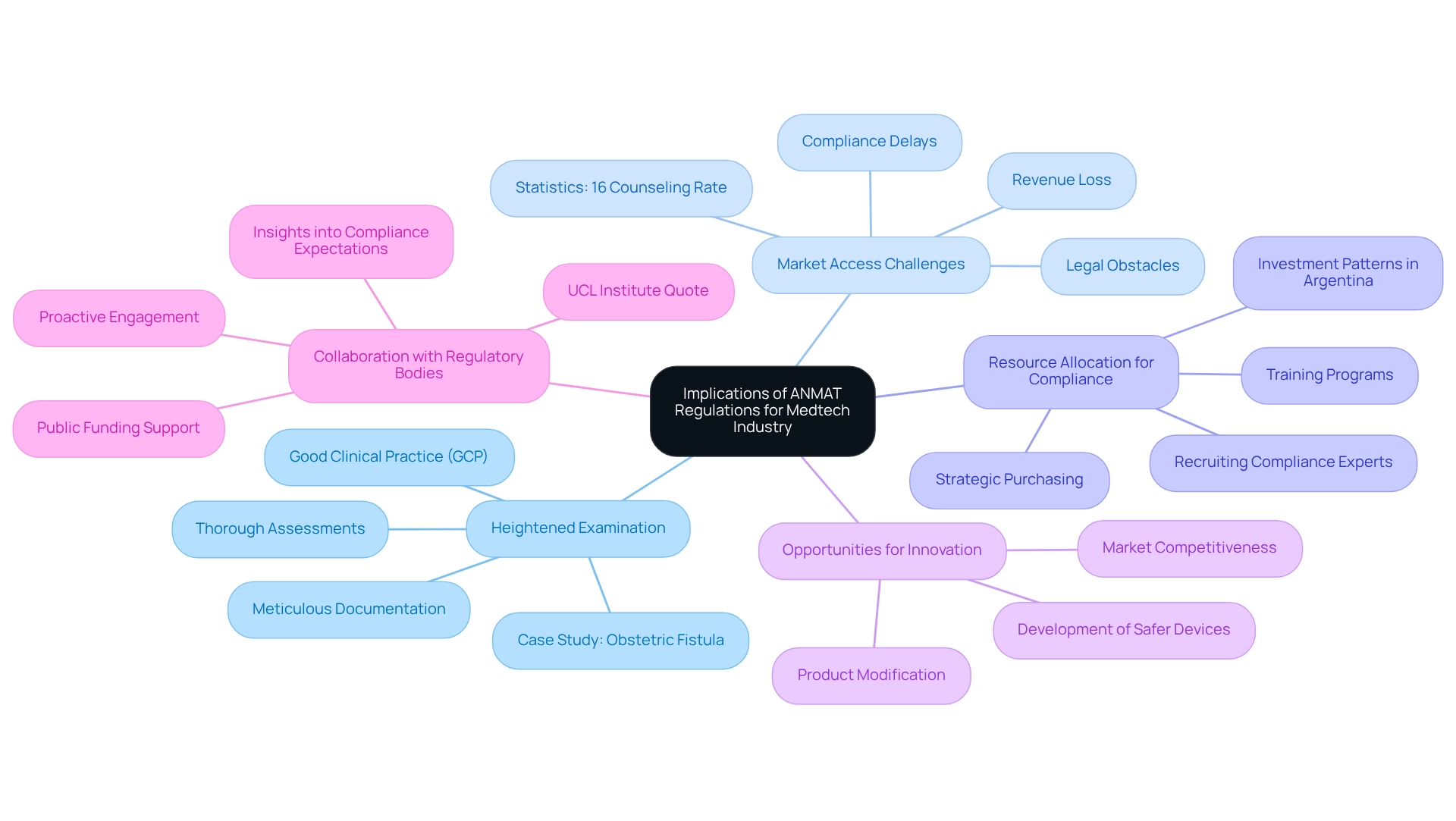
The implications of ANMAT requirements for medtech clinical trials are profound for the Medtech industry, especially in 2025, as companies navigate a complex landscape of compliance and market access. Key considerations include:
- Heightened Examination: Companies must prepare for thorough assessments of their clinical study applications. This heightened scrutiny demands meticulous documentation and strict adherence to the ANMAT requirements for medtech clinical trials and Good Clinical Practice (GCP) standards, ensuring that all aspects of the trial are transparent and accountable. The case study titled "Obstetric Fistula as an Indicator of Care Quality" illustrates the importance of quality in healthcare, highlighting systemic failures that can arise from inadequate compliance, which is particularly relevant for Medtech firms.
- Market access challenges can occur when non-compliance with ANMAT requirements for medtech clinical trials significantly delays market access for new medical devices. Such delays not only hinder business operations but can also result in lost revenue opportunities, as companies may miss critical market windows. Recent statistics suggest that compliance-related delays have turned into a pressing concern, with many firms reporting prolonged timelines for product launches due to legal obstacles. For instance, in seven low- and middle-income countries, only 16% of patients diagnosed with cardiometabolic diseases were counseled on tobacco use, reflecting broader systemic challenges that can affect compliance and market access.
- To navigate these challenges, Medtech companies are increasingly allocating resources toward compliance efforts to meet ANMAT requirements for medtech clinical trials. This involves recruiting specialized compliance affairs experts and investing in thorough training programs to ensure that all team members are well-versed in the ANMAT requirements for medtech clinical trials. Data reveals a significant increase in investment patterns among firms in Argentina, indicating a proactive approach to adhering to regulations. Furthermore, strategic purchasing can leverage quality improvements through informed funding allocation to providers, enhancing compliance outcomes.
- Opportunities for Innovation: The changing framework also offers distinctive chances for innovation. Businesses that modify their products to comply with strict legal standards can improve their competitiveness in the market. This adaptability not only fosters compliance but also drives the development of safer and more effective medical devices.
- Collaboration with Regulatory Bodies: Proactive engagement with the ANMAT requirements for medtech clinical trials can lead to smoother approval processes. Building a cooperative relationship with oversight bodies enables companies to obtain insights into compliance expectations and promotes a more efficient route to market. As emphasized by the UCL Institute for Innovation and Public Purpose, public funding should support and stipulate participation in open data repositories and collaborative research initiatives, which can be crucial in navigating regulatory landscapes.
In conclusion, a comprehensive grasp of the ANMAT requirements for medtech clinical trials is essential for Medtech companies aiming to thrive in the Argentine market and beyond. By embracing compliance as a strategic priority, companies can mitigate risks and leverage opportunities for growth and innovation. Additionally, initiatives like the Salud Mesoamerica Initiative demonstrate successful strategies in addressing health inequities, paralleling the efforts needed for compliance with ANMAT regulations.
Furthermore, partnering with bioaccess®, which boasts over 20 years of experience in Medtech and offers a customized approach to managing clinical trials, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, can ensure a successful navigation through the regulatory landscape. To learn more about how bioaccess® can assist you, please reach out for a consultation.
Conclusion
Argentina's regulatory environment, led by ANMAT, poses both challenges and opportunities for stakeholders in the Medtech sector. The agency's unwavering commitment to ensuring safety and efficacy in clinical trials is evident through its stringent requirements and ongoing efforts to enhance transparency. For researchers aiming to navigate this complex landscape successfully, understanding key regulations—such as the necessity for ethics committee approval, adherence to Good Clinical Practice, and robust safety monitoring—is crucial.
Recent developments, including streamlined approval processes and the introduction of electronic submissions, have significantly improved the efficiency of clinical trial applications. These advancements position Argentina as an attractive hub for medical technology innovation, particularly for startups eager to bring new products to market. Nevertheless, the challenges posed by lengthy approval times, complex documentation, and the need for local expertise must not be overlooked. Addressing these issues through thorough preparation, open communication with regulatory bodies, and leveraging local knowledge can greatly enhance compliance and expedite the process.
Ultimately, the evolving regulatory landscape in Argentina highlights the importance of strategic investment in compliance. Medtech companies that prioritize regulatory adherence not only mitigate risks but also unlock opportunities for innovation and market access. By collaborating with experienced partners like bioaccess®, organizations can navigate the regulatory framework more effectively, ensuring they remain competitive in an increasingly dynamic environment. Embracing these practices will be vital for fostering a thriving Medtech ecosystem in Argentina and beyond.
Frequently Asked Questions
What is ANMAT and what is its role in Argentina?
The National Administration of Medicines, Food and Medical Technology (ANMAT) is Argentina's regulatory authority responsible for ensuring the safety, efficacy, and quality of medical products, including medical devices and pharmaceuticals. It plays a crucial role in upholding national and international standards for research studies.
What are the primary responsibilities of ANMAT?
ANMAT's primary responsibilities include evaluating research study applications, conducting thorough inspections, and enforcing regulations that protect public welfare.
What changes to medical device regulations will occur in 2025?
Beginning in 2025, revisions to Argentina's medical device regulations will streamline the approval process, enhancing efficiency for businesses involved in research.
How does ANMAT ensure compliance in research studies?
ANMAT ensures compliance by proactively monitoring research studies and implementing changes aimed at increasing transparency and efficiency in the approval process.
What types of studies does bioaccess® manage?
Bioaccess® manages various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What are the key requirements for conducting clinical studies in Argentina?
Key requirements include submitting a Clinical Trial Application (CTA), obtaining ethics committee approval, adhering to Good Clinical Practice (GCP) guidelines, enforcing safety monitoring protocols, and maintaining comprehensive documentation and record keeping.
How important is ethics committee approval in the clinical trial process?
Ethics committee approval is crucial as it ensures that the study meets ethical standards and protects the rights and welfare of participants. Approximately 85% of clinical studies in Argentina successfully obtain this approval.
What is the significance of Good Clinical Practice (GCP) compliance?
Compliance with GCP guidelines is essential for maintaining data integrity and safeguarding participant rights, enhancing the credibility of research findings.
What role does safety monitoring play in clinical studies?
Safety monitoring is vital to ensure participant safety during the study, which includes timely reporting of any adverse events.
Why is it important to understand ANMAT requirements for medtech clinical trials?
Understanding ANMAT requirements is essential for the effective commencement and implementation of studies in the Medtech field, as it influences compliance obligations and the overall approval process.




