Overview
This article delves into the critical understanding of trial endpoints for medical devices, underscoring their vital role in evaluating the effectiveness and safety of medical interventions. It articulates the necessity of clearly defined primary and secondary outcomes, as well as the importance of both objective and subjective measures. Furthermore, it examines the influence of regulatory guidelines on endpoint selection, all of which are essential for ensuring successful clinical trials and producing reliable research outcomes.
Introduction
The landscape of clinical trials is evolving rapidly, particularly as the importance of endpoint selection becomes increasingly recognized. Endpoints serve as critical metrics that determine the success of medical interventions, influencing everything from trial design to regulatory approval. As we approach 2025, the stakes are higher than ever, with researchers grappling with the complexities of defining both primary and secondary endpoints that not only meet regulatory standards but also resonate with patient experiences.
This article delves into the nuances of endpoint selection, exploring the challenges, best practices, and emerging trends—including the rise of digital endpoints—that are shaping the future of clinical research. By understanding the intricacies of effective endpoint selection, stakeholders can enhance the quality and relevance of clinical trials, ultimately leading to better outcomes for patients and advancements in medical technology.
Defining Clinical Trial Endpoints: The Basics
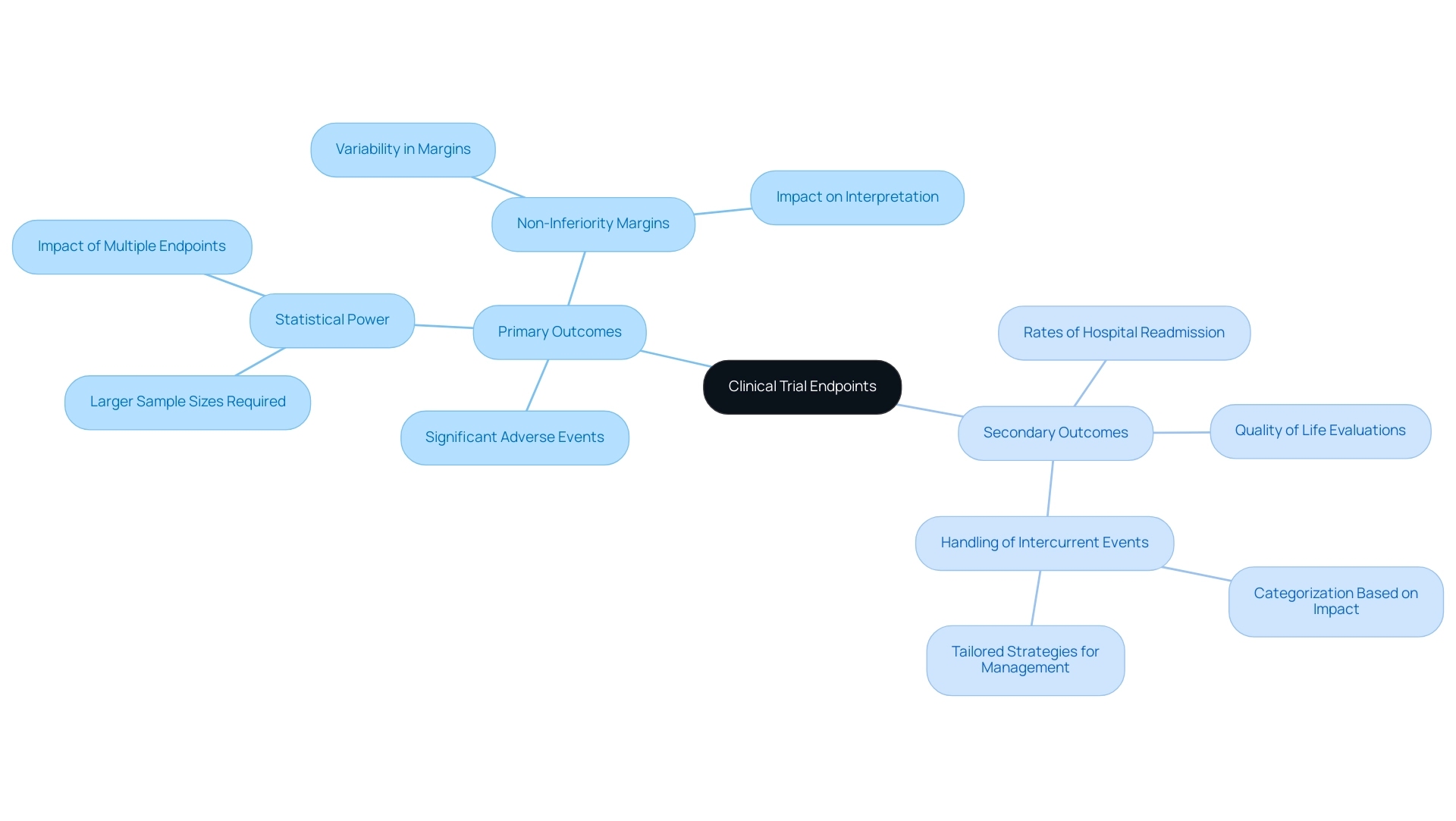
Clinical trial objectives are critical metrics that researchers utilize to assess the effectiveness of medical interventions. These access points are typically divided into two categories: primary and secondary. Primary outcomes represent the main results of interest in a study, while secondary measures provide additional insights into the intervention's effects.
For instance, in a clinical study assessing a new cardiac device, the main goal might concentrate on the frequency of significant adverse cardiac occurrences, while secondary objectives could include quality of life evaluations or rates of hospital readmission.
The significance of precisely outlining these objectives cannot be overstated, especially concerning trial endpoints for medical device evaluations. In 2025, the focus on accurate outcome definitions is crucial, as they directly affect the study's design, statistical strength, and ultimately, the analysis of findings. Trials that require all outcomes to be significantly associated with the intervention often necessitate larger sample sizes compared to those with a single primary outcome, highlighting the need for strategic planning in outcome selection.
As noted, a study requiring all outcomes to be significantly associated with the intervention has less power and necessitates larger samples compared to designs with one primary outcome.
Recent trends in clinical research, particularly in endocrinology, have shown a rise in complexity as studies evolve from simpler to more intricate indications. This shift requires a strong structure for defining and assessing targets to ensure that research remains focused and effective. The growing intricacy in these assessments highlights the necessity for detailed trial endpoints for medical devices, which can greatly influence the results of evaluations.
Furthermore, the determination of non-inferiority thresholds in studies is a vital factor that differs considerably and can profoundly affect the interpretation of findings. Thorough examination of these margins is crucial for preserving the integrity of the findings, as they can affect how results are interpreted and responded to.
By integrating insights from specialists, Wittes proposes that researchers may contemplate modifications to the main objective when the examination has secure protocols to ensure the separation of individuals involved in making such adjustments from information that could shed light on treatment impact. This emphasizes the significance of upholding strict criteria in management of endpoints.
At bioaccess®, we utilize over 20 years of experience in overseeing medical studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF). Our extensive clinical research management services include feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. This holistic approach guarantees that endpoint definitions are not only precise but also in line with the overall goals of the research, enhancing the quality and reliability of the research outcomes.
Examples, such as those addressing the handling of intercurrent events during the pandemic, illustrate the necessity of categorizing these events based on their impact on treatment adherence and outcome ascertainment. By implementing tailored strategies to manage these challenges, researchers can uphold the integrity of research objectives while adapting to unforeseen circumstances. The classification of intercurrent events enables a more refined method of preserving research integrity, guaranteeing that the distinct challenges presented by such occurrences are efficiently tackled.
In summary, a comprehensive grasp of trial endpoints for medical devices—covering their definitions, examples, and statistical implications—is essential for the successful management and assessment of medical device evaluations. This knowledge not only aids in the design of effective studies but also enhances the overall quality of medical research, particularly when supported by the specialized services offered by bioaccess®.
Types of Clinical Trial Endpoints: Objective vs. Subjective Measures
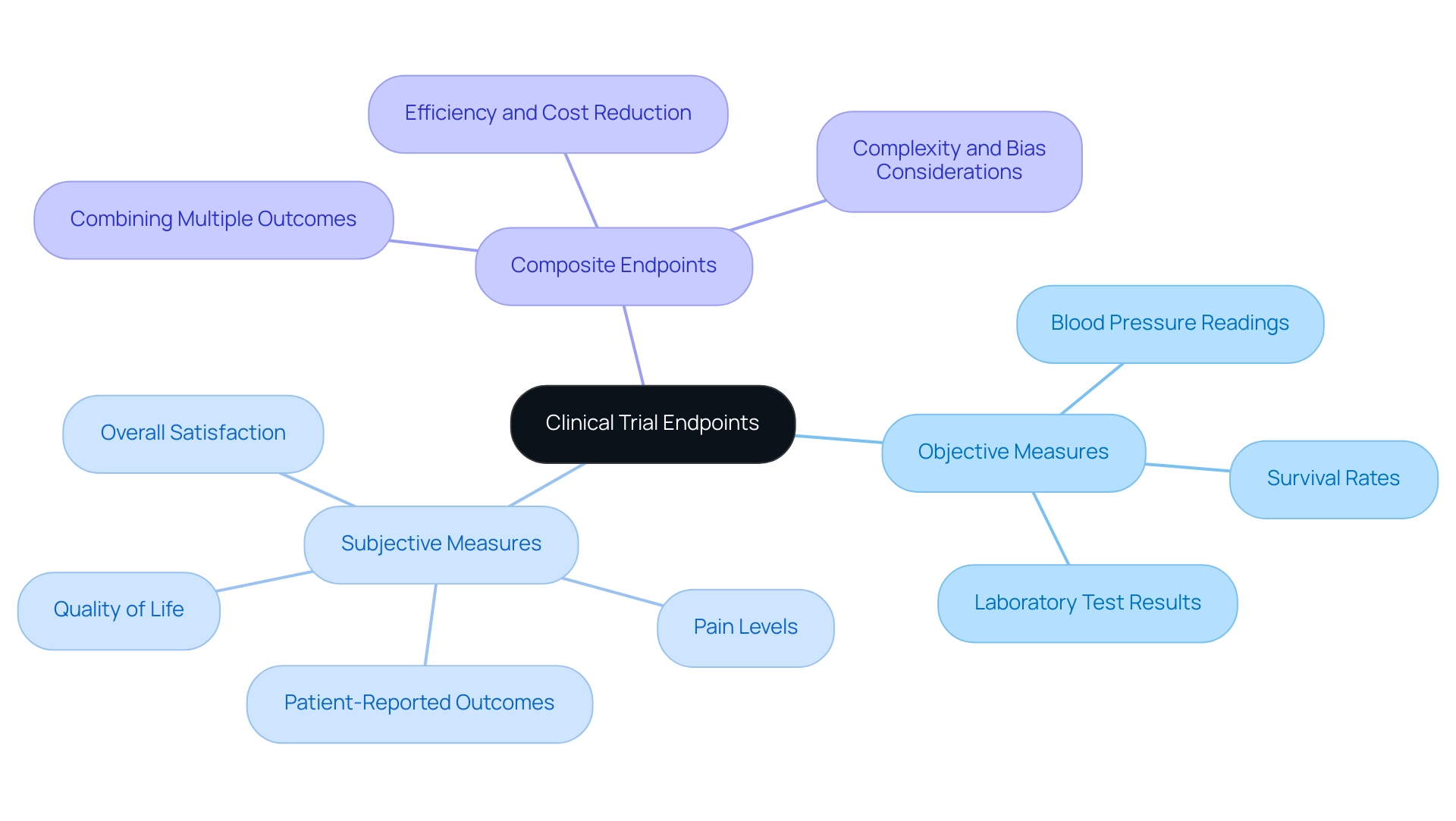
Endpoints in clinical trials are categorized into two primary types: objective and subjective. Objective measures are quantifiable and measurable, encompassing metrics such as blood pressure readings, survival rates, or laboratory test results. These access points provide concrete data that can be statistically analyzed, making them essential for evaluating the efficacy of medical devices.
Conversely, subjective measures rely on patient-reported outcomes, which include evaluations of pain levels, quality of life, and overall satisfaction with treatment. For instance, in a medical study assessing a pain management device, researchers might utilize both objective measures—such as the decrease in medication use—and subjective measures, like patient-reported pain scores. This dual approach offers a more comprehensive perspective on the device's effectiveness, integrating both medical and experiential data.
Understanding the distinction between these outcome types is crucial when planning research studies, as trial endpoints for medical devices can significantly influence the evaluation of a medical device's performance. Recent trends indicate a growing acknowledgment of the importance of patient-reported outcomes, with research demonstrating that including subjective measures can enhance the relevance of findings to actual patient experiences. A recent evaluation highlighted that studies employing both objective and subjective measures yielded more thorough insights into treatment effectiveness, ultimately leading to better-informed regulatory decisions.
Moreover, the application of composite measures, which merge multiple outcomes, has gained traction in clinical research, with an increase in their usage noted in recent analyses. While they can streamline processes and reduce costs, they also introduce complexities that necessitate careful consideration to mitigate potential biases in interpretation. A case analysis on composite endpoints illustrated that while they can enhance efficiency, their application requires a comprehensive understanding of the underlying data to ensure accurate evaluations of device efficacy.
Therefore, selecting the appropriate trial endpoints for medical devices—whether objective, subjective, or a combination of both—is vital for the success of studies in the medical device sector. With over 20 years of expertise in Medtech, bioaccess® underscores the significance of these factors in its focus on various types of research, including pilot trials, first-in-human trials, early-feasibility trials, pivotal trials, and post-market evaluations. Furthermore, bioaccess® leverages its specialized expertise and adaptability to navigate the complexities of medical research in Latin America, ensuring that each investigation is tailored to meet the unique regulatory and operational challenges of the region.
The Importance of Endpoint Selection in Clinical Trials
Choosing suitable targets is essential in research studies, as they significantly impact the trial endpoints for medical devices, which are critical for demonstrating both effectiveness and safety. Poorly selected markers can lead to inconclusive results, greatly compromising the study's success. For instance, a medical study assessing a diabetes management device that depends exclusively on subjective measures—such as patient-reported outcomes—without including objective metrics like HbA1c levels may fail to provide a thorough evaluation of the device's effectiveness. This oversight can lead to misleading conclusions about the device's impact on patient health.
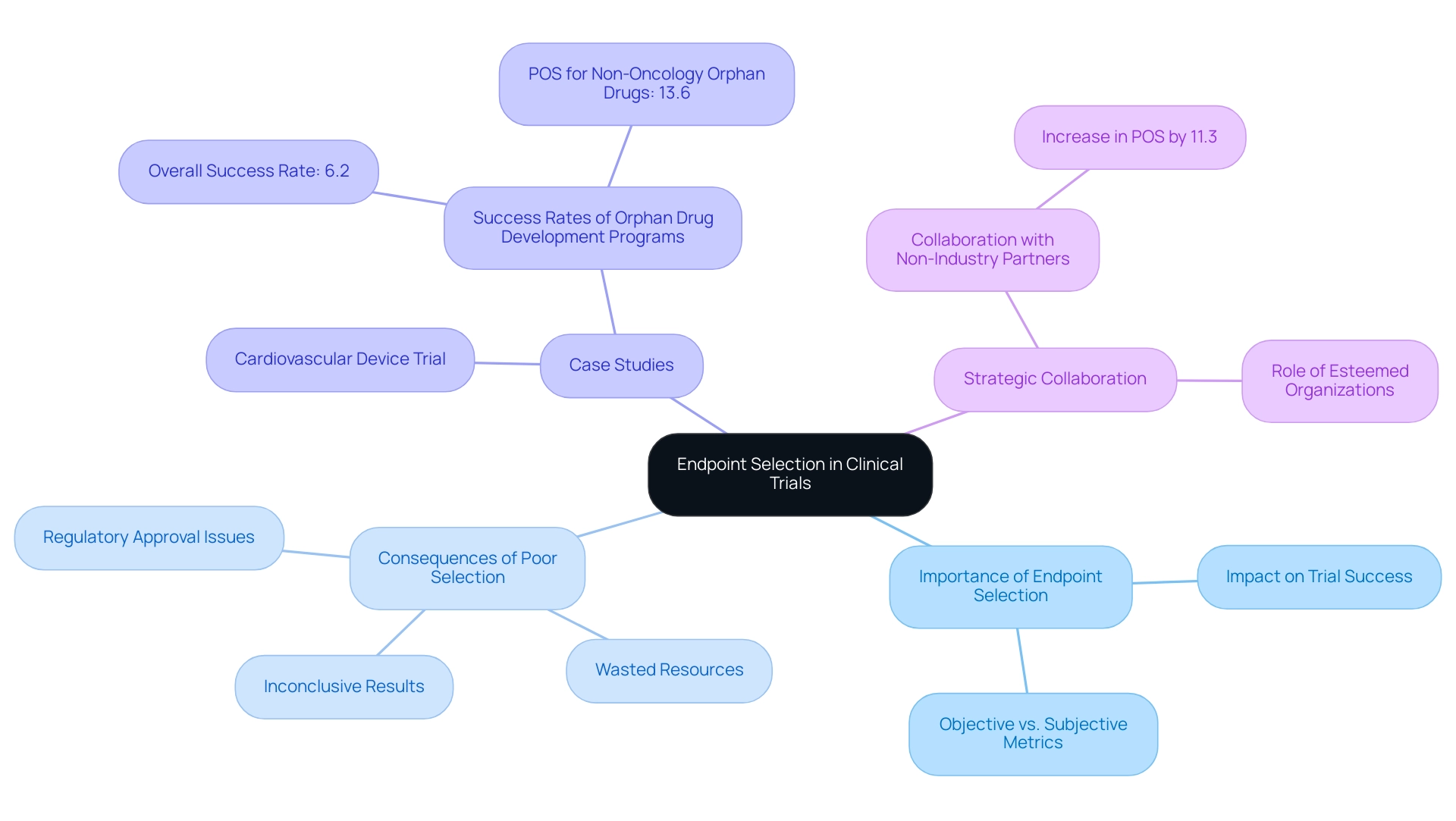
Statistics indicate that selection problems lead to a significant number of unsuccessful medical studies. In fact, studies indicate that issues related to trial endpoints for medical devices are among the leading causes of failures, underscoring the importance of meticulous planning in this area. The influence of trial endpoints for medical devices on study success rates cannot be overstated; studies with well-defined and relevant trial endpoints for medical devices are significantly more likely to achieve their objectives. Significantly, collaboration with non-industry partners has been demonstrated to enhance the likelihood of success (POS) of drug development projects by 11.3 percentage points, emphasizing the importance of strategic partnerships in research.
At bioaccess®, our comprehensive clinical study management services encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. This expertise is especially crucial in maneuvering through the intricacies of selection, ensuring that studies are structured to capture significant changes in patient health while conforming to regulatory standards. Poorly chosen trial endpoints for medical devices can lead to trials that do not adequately capture the true effects of the intervention, resulting in wasted resources and delayed access to potentially beneficial medical devices.
We specialize in managing various types of research, including Early-Feasibility Research (EFS), First-In-Human Research (FIH), Pilot Research, Pivotal Research, and Post-Market Clinical Follow-Up Research (PMCF). Our focus on these research types is essential for addressing the unique challenges presented in the medical device landscape, particularly in Latin America, where regulatory environments, such as INVIMA, play a critical role in the approval process.
Case analyses demonstrate the effects of insufficient final point selection. For example, a trial for a new cardiovascular device that failed to incorporate essential trial endpoints for medical devices related to patient survival and quality of life ultimately resulted in a lack of regulatory approval, despite promising preliminary data. Furthermore, the case study named "Success Rates of Orphan Drug Development Programs" shows that the overall success rate for orphan drug development was notably lower at 6.2%, highlighting the essential role of outcome selection in attaining favorable results. Such examples act as warning stories for researchers, emphasizing the necessity for a comprehensive understanding of implications.
As we progress into 2025, the significance of selection in medical studies, particularly regarding trial endpoints for medical devices, remains crucial. With updated projections of research success rates offering enhanced risk clarity, it is crucial for researchers to prioritize trial endpoints for medical devices as a key element in their study frameworks. By doing so, they can enhance the likelihood of successful outcomes and contribute to the advancement of medical technologies that improve patient care.
As R.M.A., a non-executive board member, observed, the participation of esteemed organizations can provide credibility to research endeavors, further emphasizing the significance of strategic collaborations in medical studies. Furthermore, the projected minimum detectable effect size (MDES) in studies utilizing ADAS-Cog would have been 4.07 points, in contrast to a change of 0.8 in the study, demonstrating the quantitative influence of outcome selection on study results.
Regulatory Considerations for Endpoint Selection
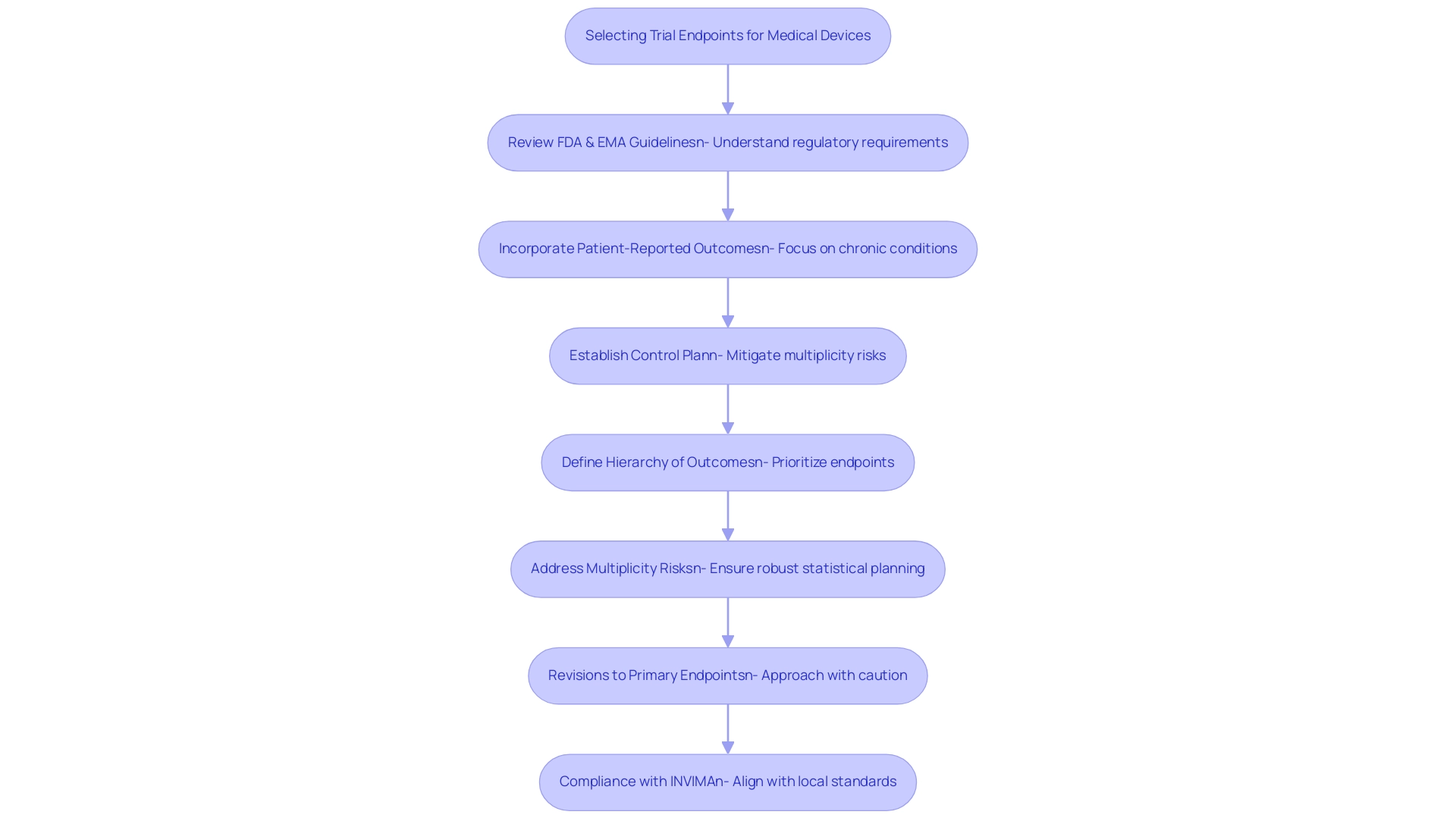
Regulatory organizations such as the FDA and EMA provide essential guidelines for selecting trial endpoints for medical devices in research studies, underscoring the necessity for outcomes to be medically significant and directly linked to patient results. The FDA, for instance, advocates for the inclusion of trial endpoints for medical devices, particularly emphasizing patient-reported outcomes in studies targeting chronic conditions. This strategy not only enriches the data collected but also yields valuable insights into how treatments impact patients' everyday lives.
In 2025, the FDA finalized guidance on managing multiple outcomes in clinical trials, highlighting the risks associated with 'multiplicity'—the potential for misleading conclusions when analyzing trial endpoints for medical devices simultaneously. This finalized guidance responds to the draft issued on January 13, 2017, and stresses the importance of establishing a control plan and a hierarchy of outcomes as trial endpoints for medical devices to mitigate these risks, ensuring that statistical planning is sufficiently robust to produce valid conclusions regarding treatment efficacy.
Moreover, revisions to main objectives should be approached with caution, as frequent changes can lead to misguided research outcomes and ultimately compromise patient care. Regulatory specialists emphasize that staying abreast of these evolving guidelines is crucial for researchers to ensure adherence and align their selection of trial endpoints for medical devices with the overarching aims of their studies. As bioaccess offers extensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—researchers can benefit from expert guidance in navigating these complex regulatory landscapes.
In Colombia, the role of INVIMA as the national regulatory authority is vital in overseeing medical device evaluations, ensuring compliance with local standards. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provides valuable insights into effectively navigating these regulations.
As Blake E. Wilson noted, "If you have any inquiries regarding how to classify the outcomes in a research study or the capacity to draw conclusions from various outcomes in a research study, or with research studies more broadly, please feel free to reach out to any of the authors of this alert or the Hogan Lovells attorney you typically collaborate with."
As the landscape of medical research continues to evolve, understanding the FDA and EMA's guidance for trial endpoints for medical devices becomes increasingly essential. By adhering to these guidelines, alongside the compliance requirements set forth by INVIMA, researchers can enhance the credibility of their studies and contribute to the advancement of medical devices that significantly improve patient outcomes.
Challenges in Selecting Effective Endpoints
Choosing suitable trial endpoints for medical devices in clinical studies presents a myriad of challenges that researchers must navigate. One of the main challenges is defining trial endpoints for medical devices that are both clinically relevant and measurable. This task is further complicated by the necessity to balance comprehensive data collection with the practicality of execution.
For instance, while a study may aim to include multiple outcomes to capture a broad range of effects, this tactic can result in heightened complexity and the risk of data overload, ultimately obstructing the clarity of results.
The complexities of outcome selection are emphasized by the fact that if the effect seen at interim analyses is minimal, a considerably larger sample size may be necessary to obtain statistically significant results. This underscores the significance of strategic planning in the design stage of research studies, especially in relation to trial endpoints for medical devices. As highlighted in the case analysis on weighted combined effect measures, utilizing such measures can enhance the understanding of clinical experiments with composite outcomes by assigning higher weights to more significant components, thereby preventing the concealment of adverse effects.
Additionally, researchers frequently encounter biases, such as guarantee-time immortal-time bias, which can complicate the interpretation of survival analyses comparing responders and non-responders. Such biases can obscure the true efficacy of a treatment, making it imperative for researchers to adopt robust methodologies that mitigate these risks. As Robert Peter Gale, a consultant, emphasizes, addressing these complexities requires a nuanced understanding of the data and its implications. Case studies illustrate the real-world implications of these challenges.
For example, studies that have faced challenges with outcome selection frequently report difficulties in obtaining regulatory approval because of ambiguous or inadequately defined results. This highlights the necessity for a well-organized framework for the selection of trial endpoints for medical devices that aligns with both medical significance and regulatory expectations. The expertise and tailored approach of bioaccess® aim to assist in advancing medical devices more quickly for companies in the Medtech sector, providing valuable support in overcoming these obstacles, including comprehensive management services such as feasibility assessments, site selection, compliance evaluations, project oversight, and specifically managing Early-Feasibility Assessments (EFA), First-In-Human Assessments (FIHA), Pilot Assessments, Pivotal Assessments, and Post-Market Clinical Follow-Up Assessments (PMCF).
Ultimately, addressing these obstacles requires careful planning and collaboration among all stakeholders involved in the research process. By promoting transparent communication and utilizing the knowledge of bioaccess®, a prominent contract research organization in Latin America, researchers can improve the chances of choosing effective targets that not only satisfy regulatory standards but also aid in the progress of medical devices.
The Rise of Digital Endpoints in Clinical Trials
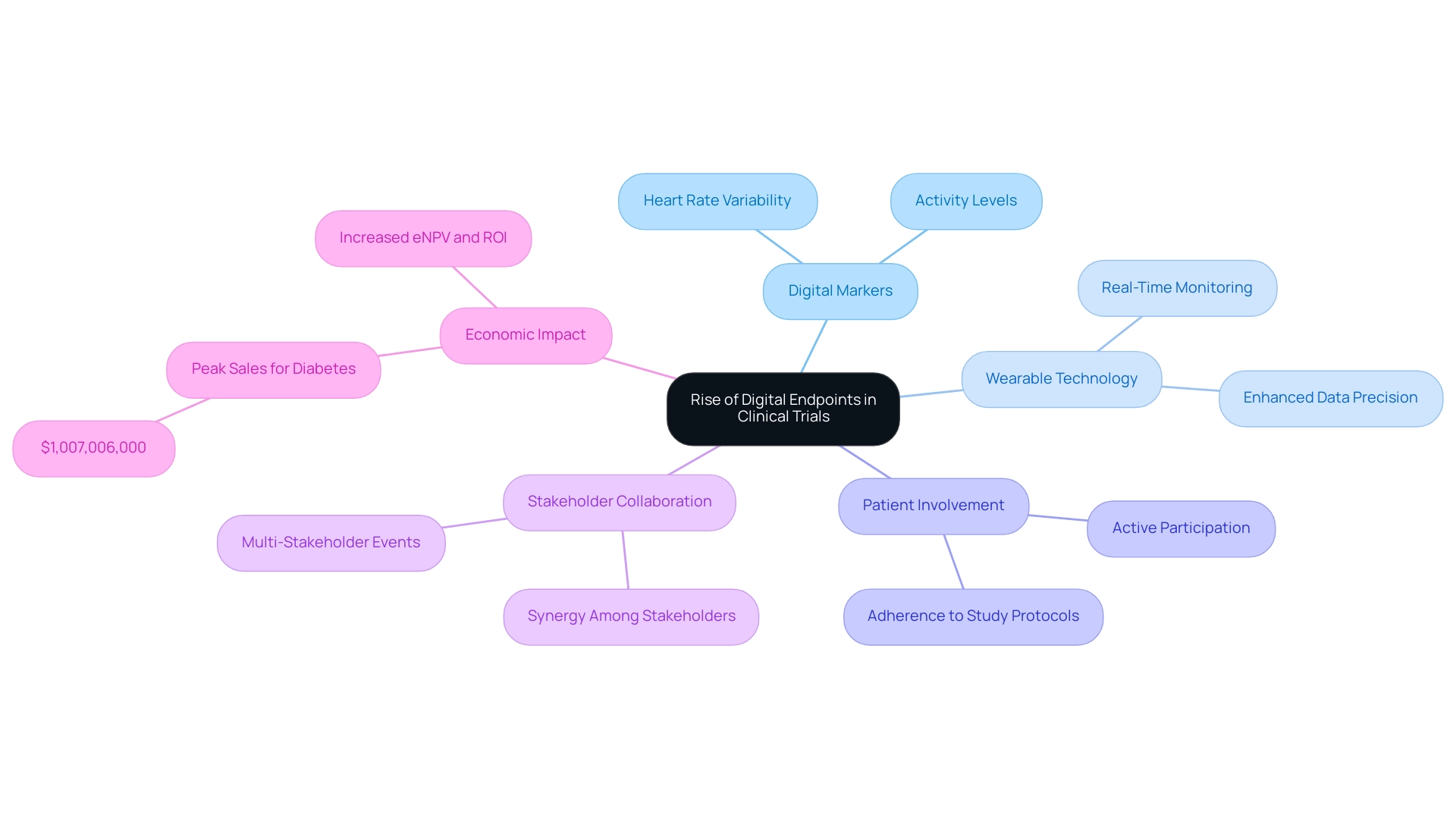
Digital markers, derived from data collected through wearable gadgets and mobile applications, are rapidly transforming the landscape of medical studies, particularly in relation to bioaccess's accelerated medical device research services in Latin America. These innovative solutions enable real-time monitoring of patient health, offering a level of detail that surpasses traditional data collection methods. For instance, in a recent cardiovascular device study overseen by bioaccess, digital metrics such as heart rate variability and activity levels were tracked using wearable sensors, showcasing the potential for enhanced data precision and increased patient involvement.
The integration of wearable technology in clinical studies has significantly influenced data collection methodologies. By facilitating continuous monitoring, these devices not only enhance adherence to study protocols but also empower patients to actively participate in their health management. This shift towards digital interfaces is supported by compelling data; for example, the adoption of digital interfaces has resulted in reduced study durations and sizes, leading to increased expected net present value (eNPV) and return on investment (ROI) across various therapeutic areas, including cardiovascular, central nervous system (CNS), and diabetes.
Notably, peak sales for diabetes indications reached $1,007,006,000, underscoring the financial impact of effective testing methodologies.
As we approach 2025, the proliferation of digital measures in research studies is expected to continue, driven by technological advancements and a growing awareness of their benefits. Experts emphasize that collaboration among stakeholders—methodologists, data analysts, and project managers—is crucial for maximizing the potential of these digital solutions. The economic feasibility of implementing digital tools has been highlighted in research conducted by Tufts CSDD and Dime, which demonstrated their role in optimizing studies and enhancing overall effectiveness.
Feedback from the recent Knowledge Exchange event revealed a broader understanding of stakeholder perspectives and underscored the need for improved guidance on organizing multi-stakeholder gatherings, further reinforcing the collaborative approach essential for the effective implementation of digital tools.
In conclusion, digital tools represent a significant advancement in research methodologies, offering a more nuanced understanding of patient outcomes and facilitating more effective medical device assessments. This is particularly evident in the determination of trial endpoints for medical devices, ultimately contributing to job creation, economic development, and healthcare improvement in the region.
Best Practices for Choosing Clinical Trial Endpoints
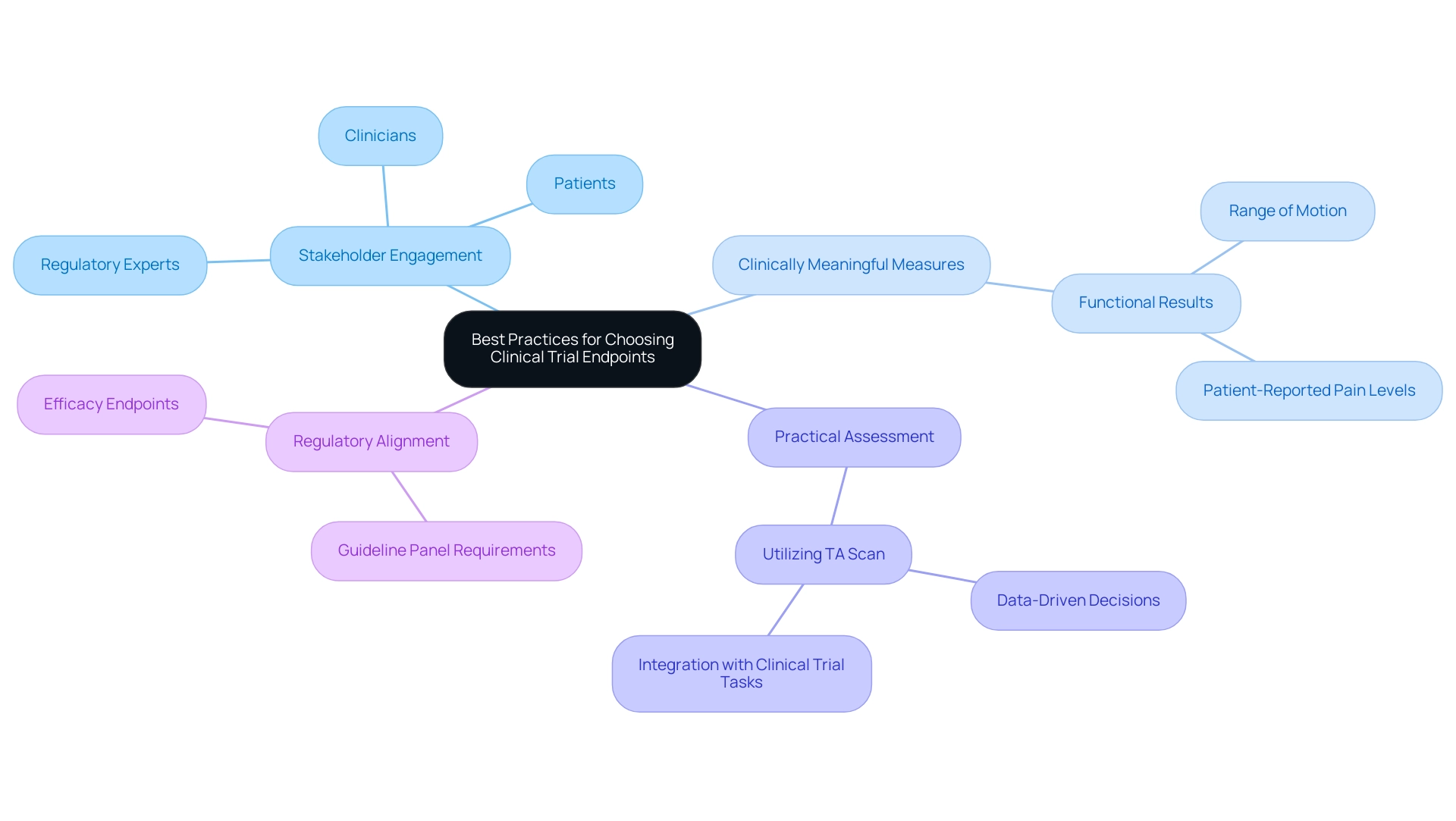
Choosing effective trial endpoints for medical devices is crucial for accurately assessing their impact. With over 20 years of experience in the Medtech sector, bioaccess® underscores the importance of adhering to various best practices to refine selection criteria. Engaging a diverse group of stakeholders—including clinicians, patients, and regulatory experts—is essential to this process. This collaboration ensures that measures capture a holistic understanding of the treatment's effects, reflecting the perspectives of those directly impacted by the device.
It is vital to prioritize measures that are clinically meaningful and closely tied to patient outcomes. For instance, in studies for orthopedic devices, measures might encompass functional results such as range of motion and patient-reported pain levels. This dual focus offers an extensive perspective on the device's effectiveness, connecting medical data with patient experiences. Furthermore, the practicality of assessing these outcomes within the study's design must be considered. Utilizing resources like Anju's TA Scan can significantly enhance selection by leveraging high-quality public information from various medical studies. This tool aids research teams in choosing superior outcomes, improving the predictability of study timelines and increasing the accuracy of outcome selection, ultimately leading to more effective research designs.
Engaging stakeholders in the outcome selection process for trial endpoints for medical devices has consistently demonstrated its ability to enhance the relevance and applicability of study results. Statistics indicate that studies incorporating stakeholder input are more likely to yield results that align with patient needs and regulatory expectations. Moreover, two methods for personalized outcomes include a sliding dichotomy based on attainable results and a values clarification tool to elicit patient preferences, providing a more nuanced understanding of optimal practices in outcome selection.
As the landscape of medical studies evolves, particularly in 2025, embracing these optimal practices will be crucial for ensuring that outcome selection is both robust and representative of the diverse viewpoints within the healthcare ecosystem. The primary objective of this review is to summarize medical and non-medical outcomes utilized in late-stage studies, while additional goals include detailing trial endpoints for medical devices and their critical features for assessing treatment impacts. As noted by Kristian Thorlund, there is now a distinction between what can be reasonably assessed in outpatient COVID-19 studies and what guideline panels require as effectiveness measures, underscoring the importance of aligning measure selection with regulatory expectations.
With bioaccess®'s comprehensive research management services in Latin America—including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies—we ensure that outcome selection is informed by extensive expertise, flexibility, and a commitment to regulatory excellence.
Case Studies: Successful Endpoint Selection in Action
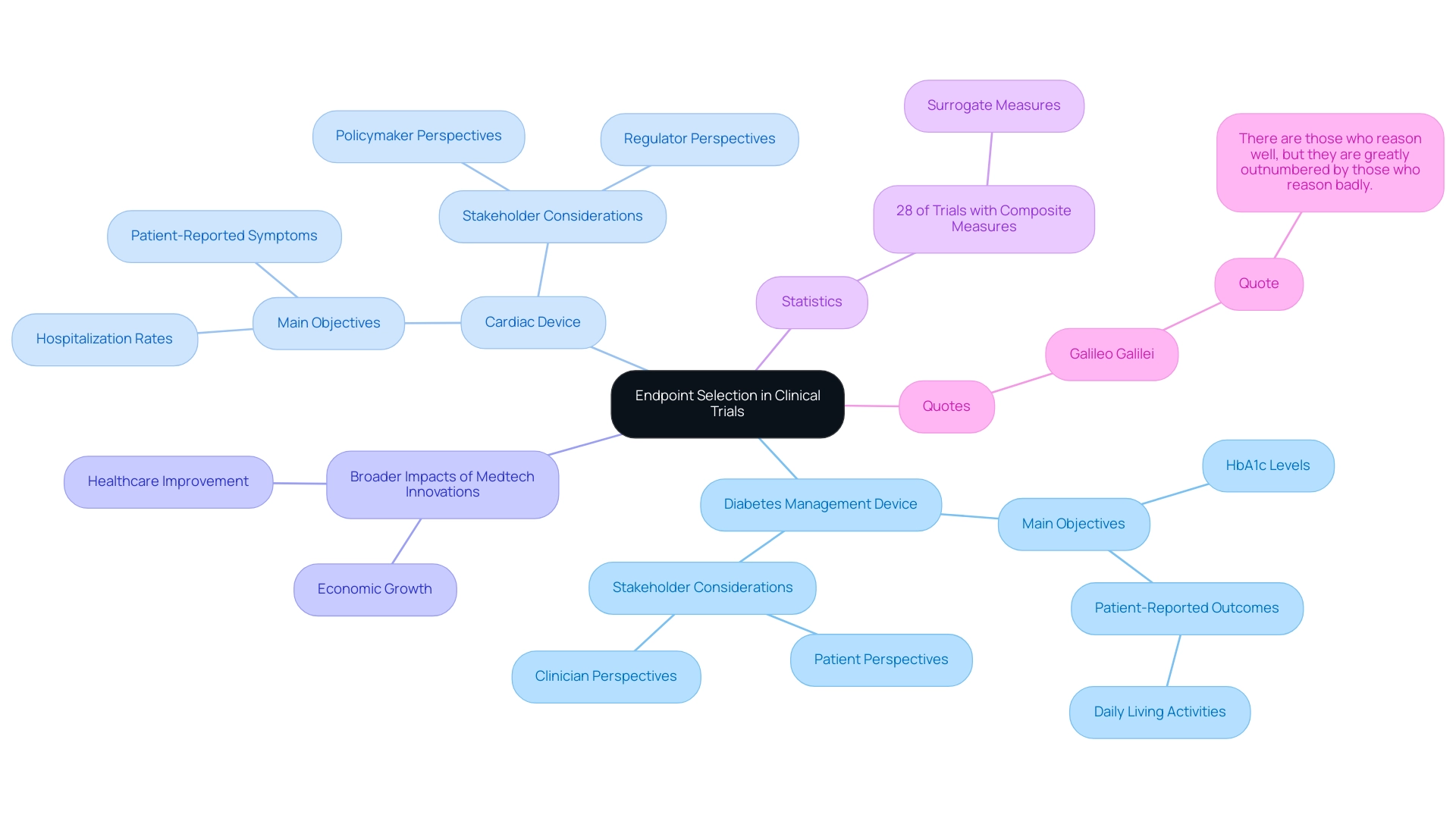
A notable case examination centered on a clinical experiment for a groundbreaking diabetes management device, where the research group thoughtfully chose a combination of main objectives. These included HbA1c levels, a critical marker for blood sugar control, alongside patient-reported outcomes that assessed daily living activities. This multifaceted approach not only confirmed the device's effectiveness in regulating blood glucose levels but also highlighted substantial improvements in patients' quality of life, showcasing the comprehensive benefits of the intervention.
Likewise, a study for a cardiac device illustrated the successful combination of both objective and subjective measures. The research measured hospitalization rates as an objective indicator of health outcomes while also incorporating patient-reported symptoms to capture the subjective experience of the patients. This dual approach offered an extensive assessment of the device's influence, guaranteeing that both clinical effectiveness and patient satisfaction were thoroughly evaluated.
These case analyses underscore the essential importance of careful outcome selection in clinical research, emphasizing that trial endpoints for medical devices can result in more significant and practical findings. By coordinating strategies with the varied requirements of stakeholders, including patients and clinicians, studies can provide insights that are not only scientifically sound but also pertinent to practical applications. As bioaccess® demonstrates through its expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot, Pivotal, and Post-Market Follow-Up Studies, understanding and integrating the perspectives of all stakeholders is essential for advancing medical devices effectively. In fact, a case study titled 'Navigating Stakeholder Perspectives in Clinical Trial Design' emphasizes the need to balance these perspectives with practical considerations such as participant burden and costs.
Additionally, statistics show that 28% of experiments utilizing a composite measure included a surrogate measure, highlighting the importance of strategic selection. As Galileo Galilei wisely noted, 'There are those who reason well, but they are greatly outnumbered by those who reason badly,' underscoring the importance of thoughtful decision-making in the context of endpoint selection. This approach not only contributes to the success of clinical trials and the establishment of trial endpoints for medical devices but also drives economic growth and healthcare improvement in local communities, showcasing the broader impact of Medtech innovations facilitated by bioaccess®, which has over 20 years of experience in the Medtech field.
Conclusion
Selecting the right endpoints in clinical trials is a pivotal factor that significantly influences the success and relevance of medical research. This article highlights the critical nature of both primary and secondary endpoints, emphasizing their role in accurately assessing the efficacy and safety of medical interventions. It underscores the importance of defining endpoints that are both clinically meaningful and aligned with patient experiences, particularly in light of the rise of digital endpoints and the integration of objective and subjective measures.
As clinical trials evolve, especially with advancements in technology and the increasing complexity of medical devices, researchers face various challenges in endpoint selection. This discussion draws attention to the necessity of strategic planning, collaboration among stakeholders, and adherence to regulatory guidelines to enhance the credibility and success rates of trials. Insights from case studies serve as powerful reminders of the tangible benefits that well-defined endpoints can bring, demonstrating their impact on patient outcomes and regulatory approvals.
In conclusion, as the landscape of clinical research approaches 2025, prioritizing meticulous endpoint selection is essential for driving advancements in medical technology. By fostering a comprehensive understanding of endpoint implications and engaging diverse perspectives, researchers can improve the quality and relevance of clinical trials. Ultimately, this commitment to effective endpoint management will not only enhance trial outcomes but also contribute to the broader goal of advancing patient care and healthcare innovations.
Frequently Asked Questions
What are clinical trial objectives and why are they important?
Clinical trial objectives are critical metrics used by researchers to assess the effectiveness of medical interventions. They are divided into primary outcomes, representing the main results of interest, and secondary outcomes, which provide additional insights into the intervention's effects. Clearly defining these objectives is crucial as they influence study design, statistical strength, and analysis of findings.
What is the difference between primary and secondary outcomes in clinical trials?
Primary outcomes are the main results of interest in a study, while secondary outcomes provide additional insights into the effects of the intervention. For example, in a study assessing a new cardiac device, the primary outcome might focus on significant adverse cardiac occurrences, while secondary outcomes could include quality of life evaluations or rates of hospital readmission.
How do trial endpoints affect the design and sample size of a study?
Trials requiring all outcomes to be significantly associated with the intervention typically need larger sample sizes compared to those with a single primary outcome. This highlights the importance of strategic planning in selecting outcomes to ensure adequate statistical power.
What recent trends have been observed in clinical research, particularly in endocrinology?
Recent trends indicate a rise in complexity in clinical research, with studies evolving from simpler to more intricate indications. This shift necessitates a strong structure for defining and assessing targets to maintain research focus and effectiveness.
What role do non-inferiority thresholds play in clinical trials?
Non-inferiority thresholds are vital factors that can significantly affect the interpretation of study findings. Thorough examination of these margins is crucial for preserving the integrity of the results and ensuring accurate interpretation.
How does bioaccess® support clinical trials?
Bioaccess® offers over 20 years of experience in managing various types of medical studies, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Medical Follow-Up Studies. Their services include feasibility assessments, site selection, compliance evaluations, and ensuring that endpoint definitions align with research goals.
What are objective and subjective endpoints in clinical trials?
Objective endpoints are quantifiable and measurable, such as blood pressure readings or survival rates. Subjective endpoints rely on patient-reported outcomes, like evaluations of pain levels or quality of life. Using both types provides a comprehensive perspective on a device's effectiveness.
Why is the inclusion of patient-reported outcomes important in clinical research?
Including patient-reported outcomes enhances the relevance of findings to actual patient experiences. Research shows that studies utilizing both objective and subjective measures yield more thorough insights into treatment effectiveness, leading to better-informed regulatory decisions.
What are composite measures in clinical research?
Composite measures combine multiple outcomes into a single measure. While they can streamline processes and reduce costs, their application requires careful consideration to avoid potential biases in interpretation.
How does bioaccess® adapt to the complexities of medical research in Latin America?
Bioaccess® leverages its specialized expertise and adaptability to navigate the unique regulatory and operational challenges in Latin America, ensuring that each investigation is tailored to meet the specific needs of the region.




