Introduction
The landscape of medical device development is rapidly evolving, necessitating a strategic approach to navigate its complexities. Central to this endeavor is the Clinical Development Plan (CDP), a vital framework that outlines the trajectory of a device from conception to market entry. This comprehensive document not only delineates the objectives and methodologies essential for clinical trials but also addresses the regulatory requirements that ensure safety and efficacy.
As the industry faces challenges such as regulatory ambiguities and resource constraints, the CDP emerges as a critical tool for:
- Aligning stakeholders
- Facilitating proactive risk management
With the increasing emphasis on patient-centric approaches and real-world evidence, understanding the nuances of a well-structured CDP is paramount for stakeholders aiming to enhance the success rates of medical devices in a competitive market.
Understanding the Clinical Development Plan for Medical Devices
A clinical development plan medical device is an essential document that outlines a strategic framework for evaluating a medical device throughout its lifecycle. This comprehensive roadmap outlines the objectives, methodologies, timelines, and compliance requirements essential for ensuring the device's safety and efficacy. Significantly, the FDA received two comments from the general public, emphasizing the importance of stakeholder engagement in the oversight process.
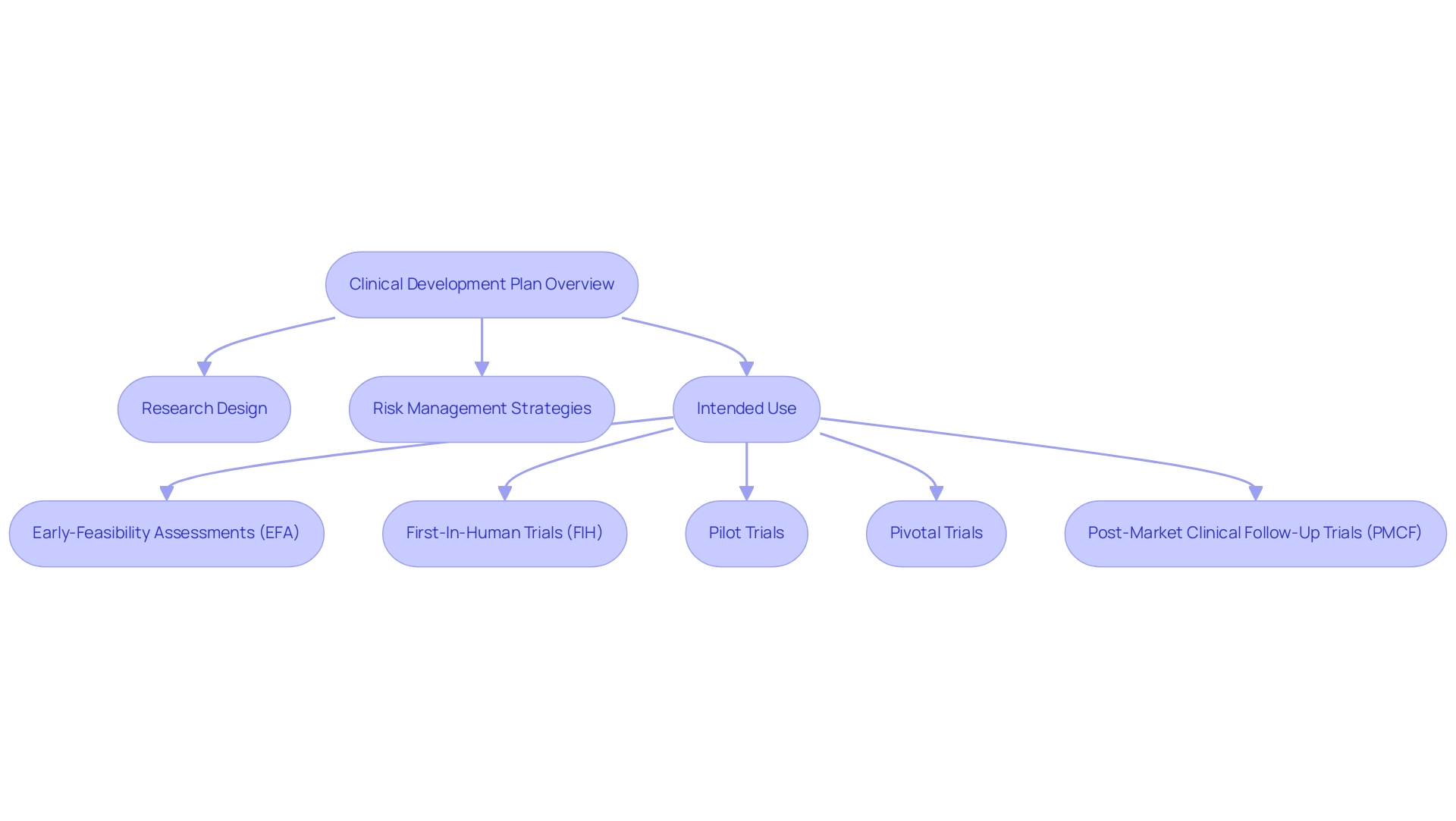
The clinical development plan for the medical device plays a crucial role in orchestrating clinical trials, ensuring alignment with the stringent standards set by oversight bodies such as the FDA and EMA. Key components of the clinical development plan medical device include:
- Research design
- Risk management strategies
- Intended use of the device
For example, bioaccess® focuses on overseeing diverse research efforts, such as:
- Early-Feasibility Assessments (EFA)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Trials (PMCF)
Utilizing more than 20 years of Medtech knowledge to navigate intricate compliance environments.
The case study of Dr. Jorge Hernando Ulloa presenting one-year data from the VenoValve® study at the Charing Cross International Symposium underscores the advancements in vascular medicine, demonstrating the efficacy of a well-structured clinical development plan medical device in achieving successful patient outcomes with minimal monitoring. By systematically addressing these elements, the clinical development plan for the medical device not only facilitates compliance with legal mandates but also enhances the likelihood of successful market access and adoption. As developments in compliance requirements evolve, particularly in 2024, the importance of a well-structured clinical development plan medical device becomes increasingly evident, serving as a vital tool for navigating the challenges inherent in clinical evaluation and device registration.
Additionally, INVIMA, as Colombia's National Food and Drug Surveillance Institute, plays a critical role in overseeing medical device regulations, ensuring compliance with local standards. As one expert noted, 'Technology is not the hurdle to more efficient drug development and registration. Agencies need to function as a global institution,' highlighting the necessity for worldwide collaboration in oversight processes.
Key Components of a Clinical Development Plan
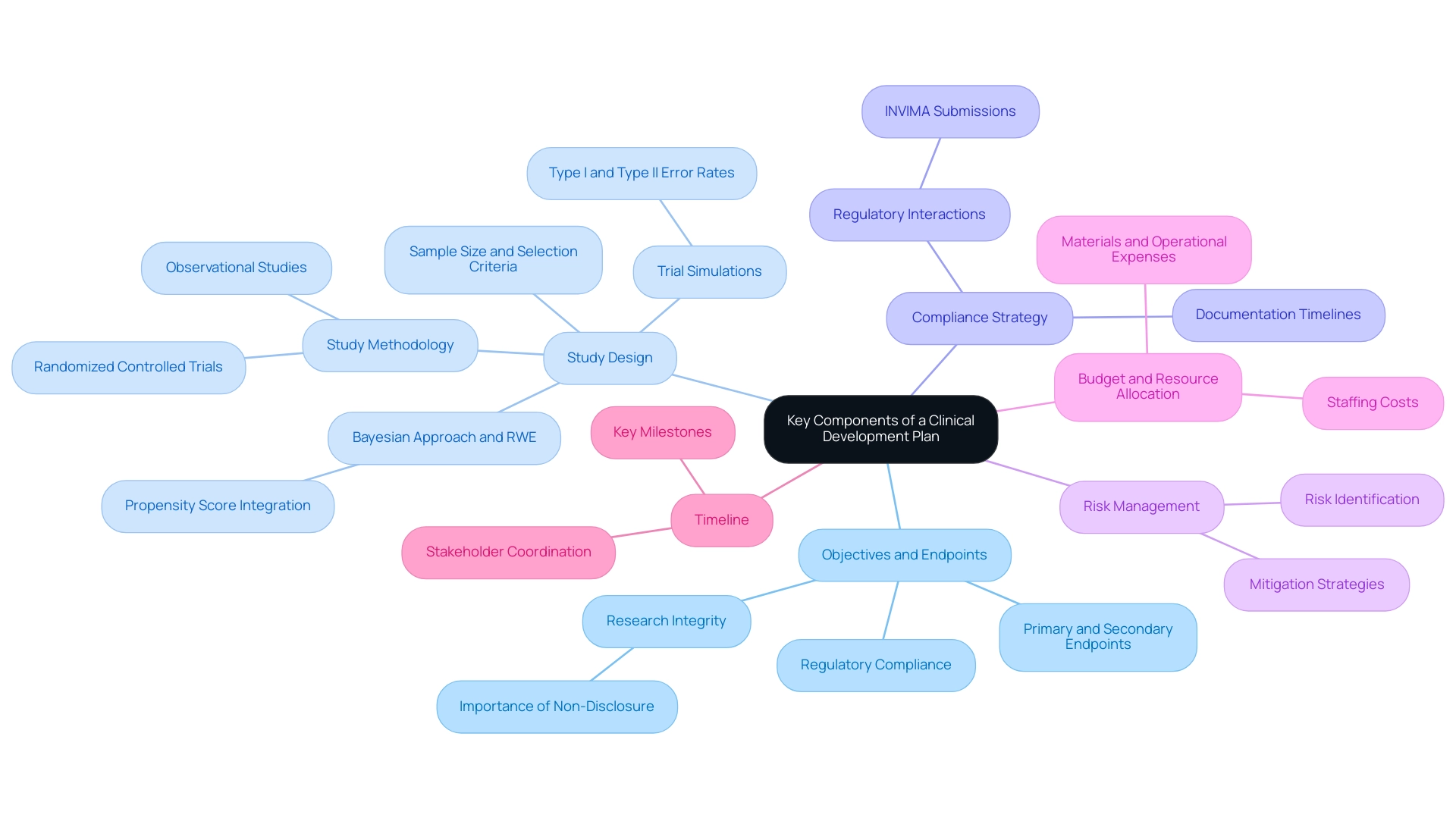
A clinical development plan medical device includes several critical components that are essential for the successful advancement of medical devices.
-
Objectives and Endpoints: Establishing clear objectives and endpoints is paramount for evaluating the device's performance.
This involves defining both primary and secondary endpoints that comply with official expectations. The emphasis on precise objectives ensures that the research aligns with the needs of regulatory bodies and stakeholders. Approximately 9,000 comments have emphasized the importance of conducting research without premature disclosure and outside pressures, highlighting the necessity for careful planning in the clinical development plan medical device.
-
Study Design: The study design outlines the methodology utilized in clinical experiments. It specifies the type of study—be it a randomized controlled experiment or an observational study—along with the sample size and participant selection criteria. Recent recommendations from the FDA advocate for simulations of trial designs at the planning stage, allowing for a thorough assessment of operating characteristics, including type I and type II error rates.
Furthermore, the Bayesian approach integrated with propensity score (PS) will play an important role in real-world evidence (RWE) synthesis, as noted by Ying Yuan, providing a contemporary perspective on evidence synthesis in medical device trials.
-
Compliance Strategy: A robust compliance strategy outlines a detailed roadmap for navigating the complexities of the oversight landscape, particularly in Latin America where bioaccess® specializes. This includes timelines for submissions and planned interactions with regulatory authorities, such as INVIMA—the Colombia National Food and Drug Surveillance Institute—ensuring that all necessary documentation is prepared and submitted in a timely manner.
-
Risk Management: Effective risk management involves the identification and assessment of potential risks associated with the device. It also encompasses strategies for mitigating these risks throughout the development process, thus safeguarding against unforeseen challenges.
-
Budget and Resource Allocation: A comprehensive budget is crucial for delineating the financial resources needed for each phase of medical development.
This includes costs related to staffing, materials, and operational expenses, ensuring that all financial aspects are thoroughly planned. The significance of resource distribution cannot be overstated, as it directly affects the feasibility and execution of the development plan.
-
Timeline: Establishing a realistic timeline that outlines key milestones within the development process is essential.
This timeline aids in guaranteeing that all stakeholders stay coordinated on project deadlines and expectations.
Furthermore, the ethical factors related to the use of placebos in research, as detailed by the Declaration of Helsinki, highlight the necessity for thorough assessment of scientific validity and ethical consequences when creating experiments involving placebos. Each of these components contributes significantly to the thoroughness, compliance, and overall success of the clinical development plan medical device, which ultimately facilitates the effective development and approval of medical devices. With the guidance of experts like Oswaldo Amaya, MD, Clinical Trial Manager at bioaccess®, companies can navigate this complex landscape effectively.
Additionally, bioaccess® provides a variety of services such as feasibility assessments, site selection, compliance evaluations, project organization, import permits, project oversight, and reporting, ensuring a thorough approach to management of research evaluations.
The Importance of a Well-Structured Clinical Development Plan
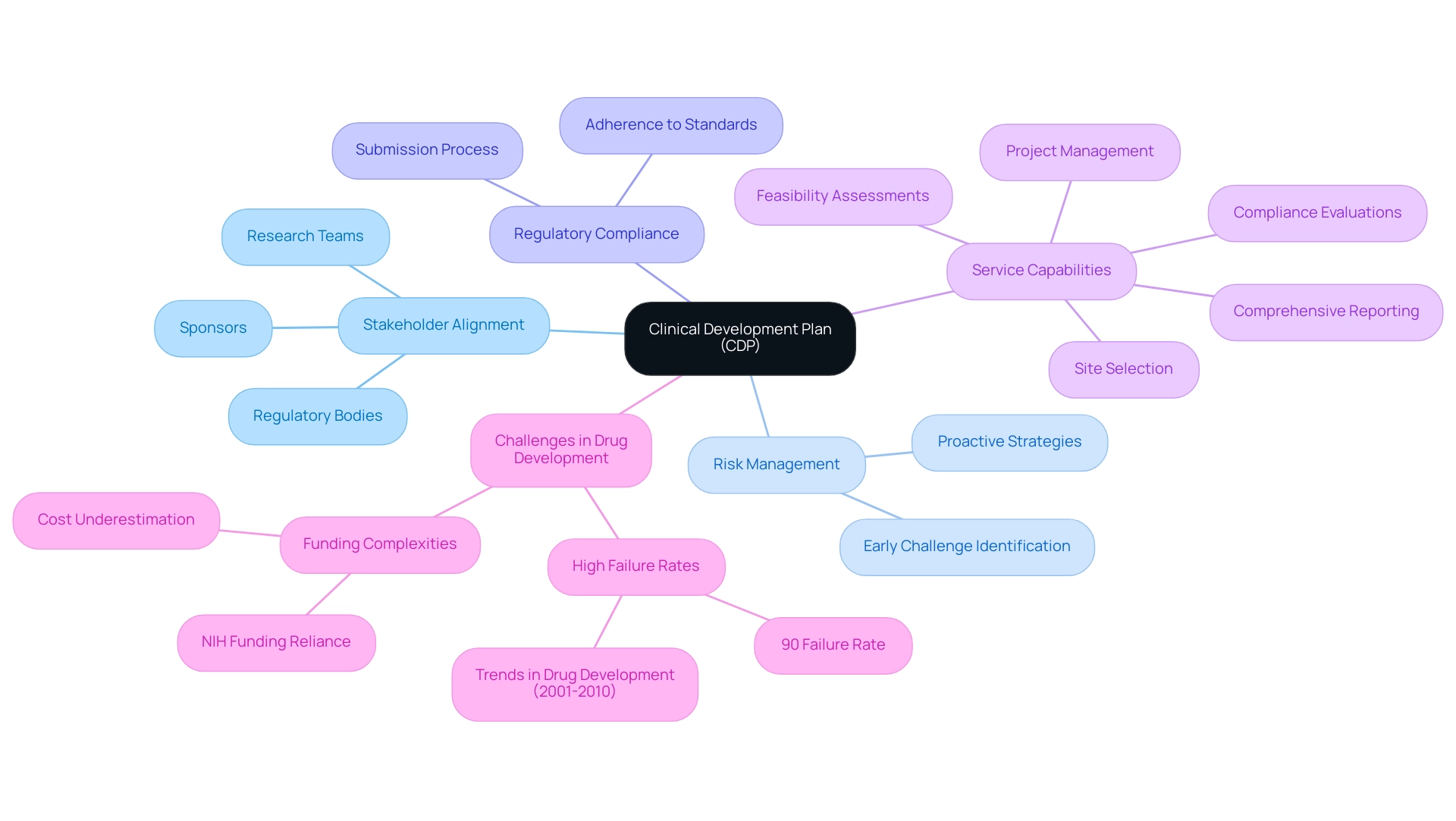
A well-organized clinical development plan medical device is essential for the success of research studies for various reasons. Primarily, it establishes alignment among all stakeholders—including sponsors, regulatory bodies, and research teams—regarding the project's objectives and methodologies. This alignment is crucial in mitigating misunderstandings and fostering effective collaboration throughout the research process.
Furthermore, a comprehensive CDP enables early identification of potential challenges, allowing for the implementation of proactive risk management strategies. This foresight is particularly important given the alarming statistic that over 90% of NIH funding for phased clinical trials is allocated through cooperative agreements or program projects, indicating a significant reliance on structured frameworks. However, limitations in the research include potential underestimation of NIH costs and challenges in comparing NIH and industry spending due to different methodologies, which highlights the complexities involved in funding and resource allocation.
Furthermore, a well-defined plan simplifies the submission process by providing authorities with clear and concise information regarding the design and objectives of the research. Our service capabilities further enhance this process through:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Preparation
- Import permits
- Project management
- Comprehensive reporting on progress, inventory, and serious and non-serious adverse events
This ensures that each phase of the research aligns with regulatory standards and best practices. The case study titled 'Trends in Drug Development (2001-2010)' illustrates the challenges encountered in advancing through trial phases, noting that only one compound achieved approval from projects initiated after 2001.
This underscores the necessity of a robust clinical development plan medical device in effectively navigating the complexities of drug development. As emphasized by Duxin Sun, such a persistent high failure rate raises several questions: Why does 90% of drug development fail despite the implementation of many successful strategies over the past several decades? This highlights the significance of a carefully designed clinical development plan medical device, which serves as a foundation for successful research, promotes smoother project execution, and greatly increases the chances of compliance approval while fostering economic growth and healthcare advancement through global collaboration.
Common Challenges in Developing a Clinical Development Plan
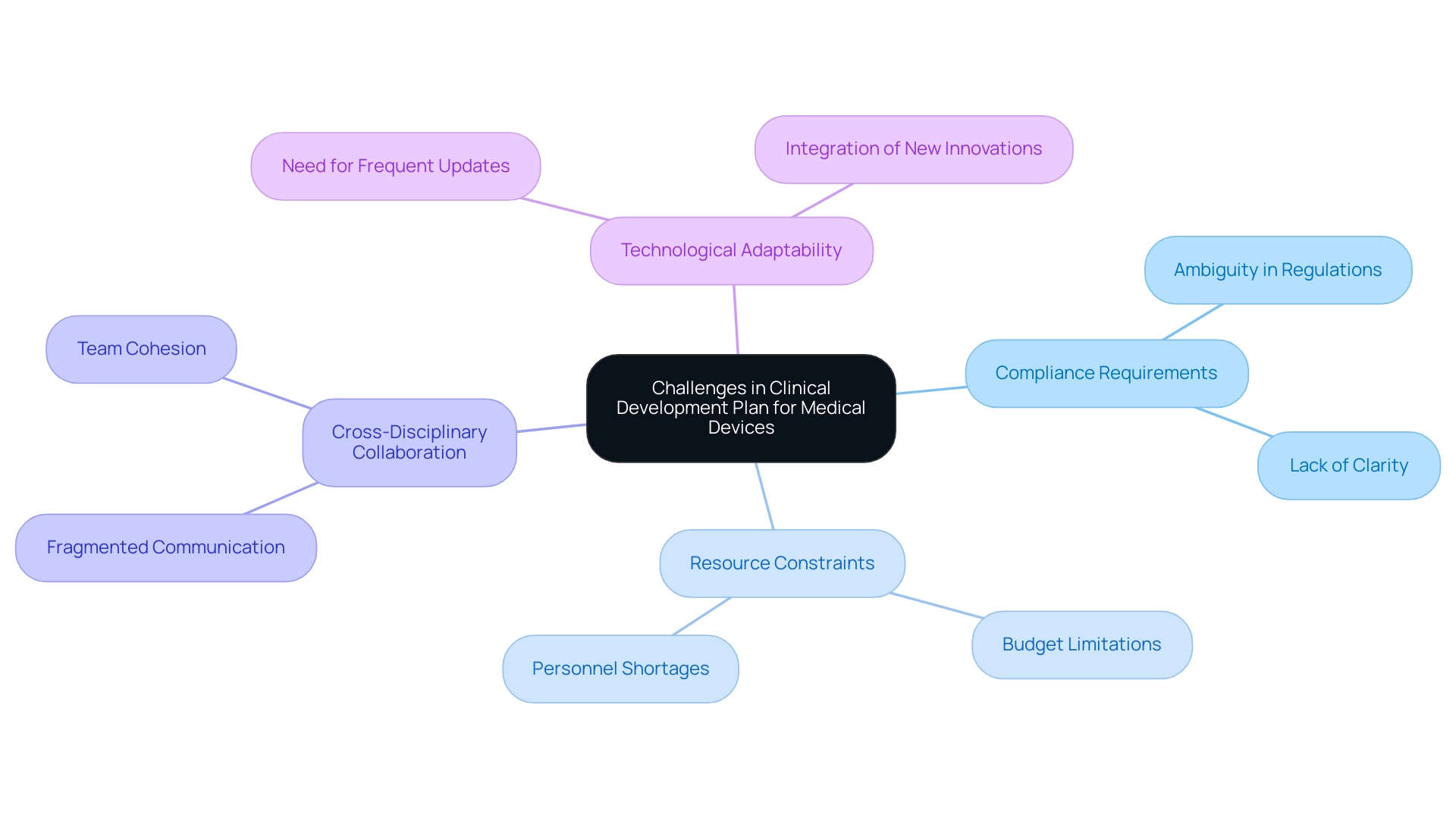
The clinical development plan medical device presents a myriad of challenges that can impede progress and success. One critical issue is the ambiguity surrounding compliance requirements, which often leads to discrepancies between the CDP and expectations. This lack of clarity can lead to significant setbacks in the clinical development plan for medical devices, as 40% to 50% of failures in drug development arise from insufficient efficacy—an insight that emphasizes the need for a robust clinical development plan for medical devices.
Our extensive management services for research tackle these challenges through:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- The import permit and nationalization of investigational devices
This ensures adherence to guidelines. Moreover, resource constraints, including budget limitations and personnel shortages, frequently hinder the comprehensive execution of the clinical development plan for the medical device. For instance, the case study titled 'Trial Design and Biomarkers' highlights how even with optimized trial designs and dosing regimens, a high proportion of drug candidates still fail, suggesting that methodologies may need reevaluation.
Effective cross-disciplinary collaboration is crucial; without cohesive communication among medical, compliance, and operational teams, the development process risks becoming fragmented. Additionally, the rapidly evolving technological landscape demands that the clinical development plan for medical devices be adaptable, requiring frequent updates to remain aligned with new regulatory guidelines and innovations. The introduction of the STAR system exemplifies how advancements in technology can enhance drug optimization efficiency and improve study success rates, addressing some of the challenges faced in development.
As mentioned by industry experts such as Chinmaya Sadangi, grasping these challenges and applying best practices can lead to more effective development plans. Furthermore, the role of INVIMA as a Level 4 health authority in Colombia is crucial, as it oversees regulatory functions and medical device classification, adding an additional layer of complexity that necessitates a thorough understanding of local regulations. Tackling these challenges proactively can greatly enhance the compliance and overall effectiveness of the clinical development plan medical device, establishing a solid foundation for successful medical studies.
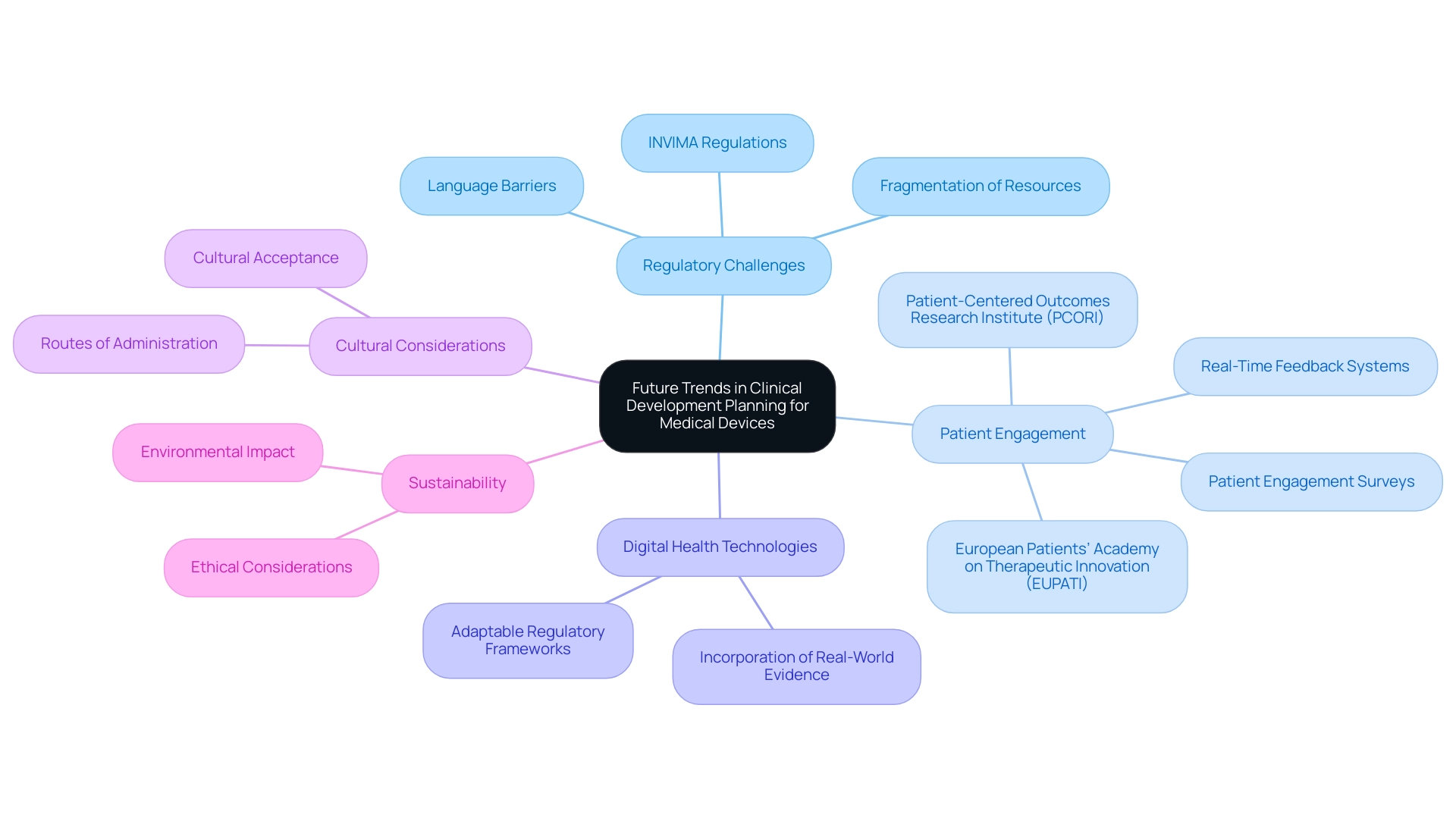
Future Trends in Clinical Development Planning for Medical Devices
The medical device industry is undergoing a transformative evolution, marked by several key trends that are redefining the clinical development plan for medical devices. A significant challenge for Medtech companies, especially in Latin America, is bridging the gap between medical research and innovation. This is worsened by regulatory obstacles, language barriers, and the fragmentation of resources, all of which impede smooth cooperation between Latin American hospitals and US research clients.
A crucial aspect of this landscape is INVIMA, the Colombian National Food and Drug Surveillance Institute, which plays a vital role in overseeing medical device regulations and classifications, influencing how assessments are conducted in the region. Collaborations like the one between Greenlight Guru and bioaccess™ are crucial in speeding up Medtech advancements and research in the region, illustrated by PAVmed's First-In-Human study in Colombia.
As the sector moves towards patient-focused strategies, there is a growing focus on engaging patients early in the development process through technological platforms and feedback systems. bioaccess® utilizes specific methodologies, such as patient engagement surveys and real-time feedback systems, to ensure that patient perspectives are integrated into development. This trend is reflected in a six-fold increase in searches for 'patient-centered' on PubMed over the past 12 years, highlighting the growing importance of patient perspectives.
Organizations such as the European Patients’ Academy on Therapeutic Innovation (EUPATI) and the Patient-Centered Outcomes Research Institute (PCORI) are at the forefront of building trust in trials, aiding recruitment, and encouraging adherence through active patient participation.
Furthermore, advancements in digital health technologies are propelling the incorporation of real-world evidence into the clinical development plan for medical devices, allowing for more thorough assessments of device performance in real-world settings. Regulatory agencies, including INVIMA, are responding with adaptable frameworks for evaluations, which could expedite approval processes and accommodate diverse patient needs.
Cultural considerations regarding routes of administration are becoming increasingly critical, as certain methods may not be accepted across various cultural contexts. Additionally, the growing emphasis on sustainability and ethical considerations is set to shape the clinical development plan for medical devices, ensuring they are both effective and responsible. Keeping pace with these trends is essential for stakeholders aiming to ensure the relevance and effectiveness of their clinical development plan for medical devices, which ultimately benefits local economies through job creation, economic growth, and healthcare improvement.
Conclusion
A well-structured Clinical Development Plan (CDP) is essential for navigating the complexities of medical device development. It serves as a strategic framework that aligns stakeholders, outlines objectives, and establishes methodologies necessary for successful clinical trials. By addressing regulatory requirements and emphasizing proactive risk management, the CDP enhances the likelihood of achieving safety and efficacy benchmarks, thereby facilitating smoother market entry.
The critical components of a CDP include:
- Clear objectives
- Robust study designs
- Regulatory strategies
- Effective risk management
These components collectively contribute to its efficacy. As highlighted, the integration of real-world evidence and patient-centric approaches is becoming increasingly vital in the evolving landscape of medical device development. The collaborative efforts among stakeholders, regulatory bodies, and clinical teams are crucial for overcoming the inherent challenges and ensuring compliance with both local and global standards.
In conclusion, as the medical device industry continues to evolve, the significance of a meticulously crafted CDP cannot be overstated. It not only serves as a cornerstone for successful clinical trials but also fosters innovation and collaboration across the healthcare ecosystem. Embracing these frameworks will ultimately drive advancements in patient care, regulatory efficiency, and economic growth, ensuring that medical devices reach the market effectively and responsibly.
Frequently Asked Questions
What is a clinical development plan for a medical device?
A clinical development plan for a medical device is a strategic document that outlines the framework for evaluating a medical device throughout its lifecycle, including objectives, methodologies, timelines, and compliance requirements to ensure safety and efficacy.
Why is stakeholder engagement important in the clinical development plan?
Stakeholder engagement is crucial as it helps in the oversight process, ensuring that the clinical development plan aligns with the expectations of regulatory bodies like the FDA and EMA.
What are the key components of a clinical development plan for a medical device?
Key components include research design, risk management strategies, and intended use of the device. It also involves establishing clear objectives and endpoints, study design, compliance strategy, risk management, budget and resource allocation, and a realistic timeline.
What types of trials are included in the clinical development plan?
The clinical development plan may include Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF).
How does the clinical development plan facilitate compliance?
It systematically addresses legal mandates and compliance requirements, enhancing the likelihood of successful market access and adoption of the medical device.
What role does INVIMA play in the clinical development plan?
INVIMA, Colombia's National Food and Drug Surveillance Institute, oversees medical device regulations and ensures compliance with local standards.
What is the significance of establishing objectives and endpoints in the clinical development plan?
Clear objectives and endpoints are essential for evaluating the device's performance and ensuring alignment with regulatory expectations and stakeholder needs.
How does study design factor into the clinical development plan?
Study design outlines the methodology for clinical experiments, including the type of study, sample size, and participant selection criteria, which are critical for the validity of the research.
What is the importance of a compliance strategy in the clinical development plan?
A robust compliance strategy provides a roadmap for navigating regulatory complexities, ensuring timely submissions and interactions with authorities.
Why is budget and resource allocation critical in the clinical development plan?
A comprehensive budget outlines the financial resources needed for each development phase, affecting the feasibility and execution of the plan.
How does the timeline contribute to the clinical development plan?
A realistic timeline outlines key milestones, helping to coordinate project deadlines and expectations among stakeholders.
What ethical considerations are involved in the clinical development plan?
Ethical factors related to the use of placebos in research must be assessed for scientific validity and ethical consequences, following guidelines like the Declaration of Helsinki.

