Overview
The article addresses best practices for innovating medical device trials in Brazil, underscoring strategies that are essential for success within this intricate regulatory landscape. It highlights the critical role of:
- Collaboration among stakeholders
- Effective patient recruitment and retention strategies
- The integration of advanced technologies
These elements are vital to enhance trial efficiency and ensure that innovations are in alignment with local healthcare needs.
Introduction
Brazil's medical device market represents a vibrant and rapidly evolving landscape, emerging as one of the largest in Latin America. The healthcare system integrates both public and private sectors, leading to a rising demand for innovative medical technologies. This demand is driven by government initiatives aimed at enhancing quality and accessibility. As societal attitudes shift and the middle class expands, new opportunities arise, particularly in sectors previously dominated by specific demographics, such as aesthetic treatments.
However, navigating the complexities of clinical trials in this diverse environment presents unique challenges. These challenges range from stringent regulatory requirements to cultural nuances in patient recruitment. Understanding these dynamics is crucial for stakeholders aiming to align their innovations with local healthcare priorities and ensure the successful advancement of medical devices in Brazil.
Understanding the Brazilian Medical Device Landscape
Brazil's healthcare device market ranks among the largest in Latin America, characterized by a diverse array of products and increasingly propelled by medical device trial innovation. The nation's healthcare system, encompassing both public and private sectors, plays a pivotal role in shaping the adoption of healthcare technologies. Recent government initiatives aimed at enhancing healthcare quality and accessibility, including significant investments in infrastructure and healthcare programs, have further fostered an environment conducive to medical device trial innovation in Brazil.
Understanding local market dynamics is essential for effective study design. This entails recognizing the preferences of healthcare providers and patients, influenced by shifting societal attitudes and economic factors. For instance, the burgeoning middle class in Brazil is driving demand for advanced healthcare tools, evidenced by the rising interest in aesthetic procedures among men—a sector historically dominated by women.
This shift, highlighted by the increasing male participation in cosmetic procedures, not only expands the target audience but also reflects broader trends in consumer preferences that stakeholders must consider. Moreover, Brazil's unique demographic landscape, marked by a large and diverse population with varying health needs across regions, presents both opportunities and challenges for medical device trial innovation. Stakeholders are urged to leverage this understanding to align their innovations with local healthcare priorities and patient needs, ensuring that medical device trial innovation in Brazil is both effective and relevant to the communities they aim to serve.
In this context, bioaccess® offers comprehensive trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate the complexities of the Brazilian market by harnessing medical device trial innovation, facilitating rapid clinical studies for healthcare products that contribute to job creation, economic development, and enhancement of health services in the region. By doing so, they can enhance the likelihood of positive outcomes and accelerate the advancement of healthcare products in the market.
Bioaccess®'s adaptability and expert insights further ensure that studies are tailored to meet the specific needs of the local healthcare landscape.
Challenges in Conducting Medical Device Trials
Medical device trial innovation in Brazil encounters a unique set of challenges that necessitate meticulous management during healthcare equipment evaluations. The Brazilian Health Regulatory Agency (ANVISA) imposes stringent requirements, often significantly prolonging approval processes and delaying the initiation of medical device trials. As of 2025, the average timeline for ANVISA's approval of medical device studies is a pressing concern, as it can extend well beyond several months, thereby impacting the overall research timeline.
Furthermore, the need for local ethical approvals and compliance with Good Clinical Practice (GCP) introduces additional layers of complexity to medical device trial innovation in Brazil. Researchers frequently face logistical hurdles, including the demand for comprehensive documentation and adherence to diverse regulatory standards. These challenges are compounded by difficulties in patient recruitment, which may stem from cultural differences and varying levels of awareness regarding research studies, particularly within the context of medical device trial innovation in Brazil.
The registration of research studies is increasingly recognized as a scientific, ethical, and moral obligation, especially in the realm of medical device trial innovation in Brazil. This underscores the importance of conducting studies with integrity and respect for participants. To effectively navigate these challenges, proactive planning is crucial. Engaging local experts and stakeholders early in the process can streamline approvals and enhance recruitment strategies, which are vital for the success of medical device trial innovation in Brazil.
For instance, recent initiatives have highlighted the importance of gender-affirming therapies, as evidenced by COFEPRIS's approval of a study focusing on hormone therapy interactions with HIV medications in trans women. COFEPRIS asserts that 'gender-affirming therapy is important because it contributes to better quality of life and adherence to HIV treatments in trans women,' emphasizing the necessity for inclusive approaches that acknowledge the diverse demographics of participants. In this context, bioaccess® provides comprehensive clinical study management services, encompassing feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
With over 20 years of experience in Medtech, bioaccess® specializes in various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). This expertise is essential for managing the regulatory intricacies and logistical challenges inherent in conducting healthcare equipment assessments in Brazil. A strategic approach, emphasizing early collaboration with local resources and a thorough understanding of the regulatory landscape, is imperative for advancing medical device trial innovation in Brazil.
Navigating Regulatory Compliance in Brazil
Navigating Brazil's regulatory environment necessitates a comprehensive understanding of ANVISA's guidelines and the recent legislative changes impacting medical device trial innovation. The enactment of Law No. 14874/2024 has significantly streamlined the approval process; however, strict adherence to both local and international standards remains paramount.
To foster medical device trial innovation in Brazil, researchers must ensure their studies comply with Good Clinical Practice (GCP) and that all requisite documentation is meticulously prepared to prevent delays. Importantly, new registration requests by supporting institutions are prohibited within 12 months following cancellation, highlighting the critical nature of compliance and the repercussions of non-adherence. Furthermore, should ethics committee activities be suspended, it is essential to notify CONE to guarantee the continuity of protocol processing. Brazil's commitment to the Nagoya Protocol on Access and Benefit-sharing also impacts research involving non-human genetic resources, which is vital for medical device trial innovation.
Collaborating with regulatory consultants and legal experts can greatly enhance interactions with ANVISA, a crucial element for medical device trial innovation in Brazil, enabling proactive identification and resolution of potential compliance challenges. Additionally, implementing regular training sessions and updates on regulatory changes is vital to keep all team members informed and equipped to adeptly navigate the evolving landscape, particularly given the significance of medical device trial innovation in Brazil. Each ethics committee must establish written standard operating procedures (SOPs) outlining their review processes, meeting schedules, and responsibilities concerning research protocols. This standardization ensures consistency and accountability in assessing medical device trial innovation across institutions.
Producers intending to promote a healthcare product in Brazil must first ascertain the risk category of the item, as emphasized by expert Katherine Ruiz, underscoring the necessity for comprehensive knowledge and adherence to regulatory stipulations, especially regarding medical device trial innovation. bioaccess® distinguishes itself as a leading Contract Research Organization, offering extensive management services for studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), along with feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. This ensures an efficient process for health-related evaluations in Brazil.
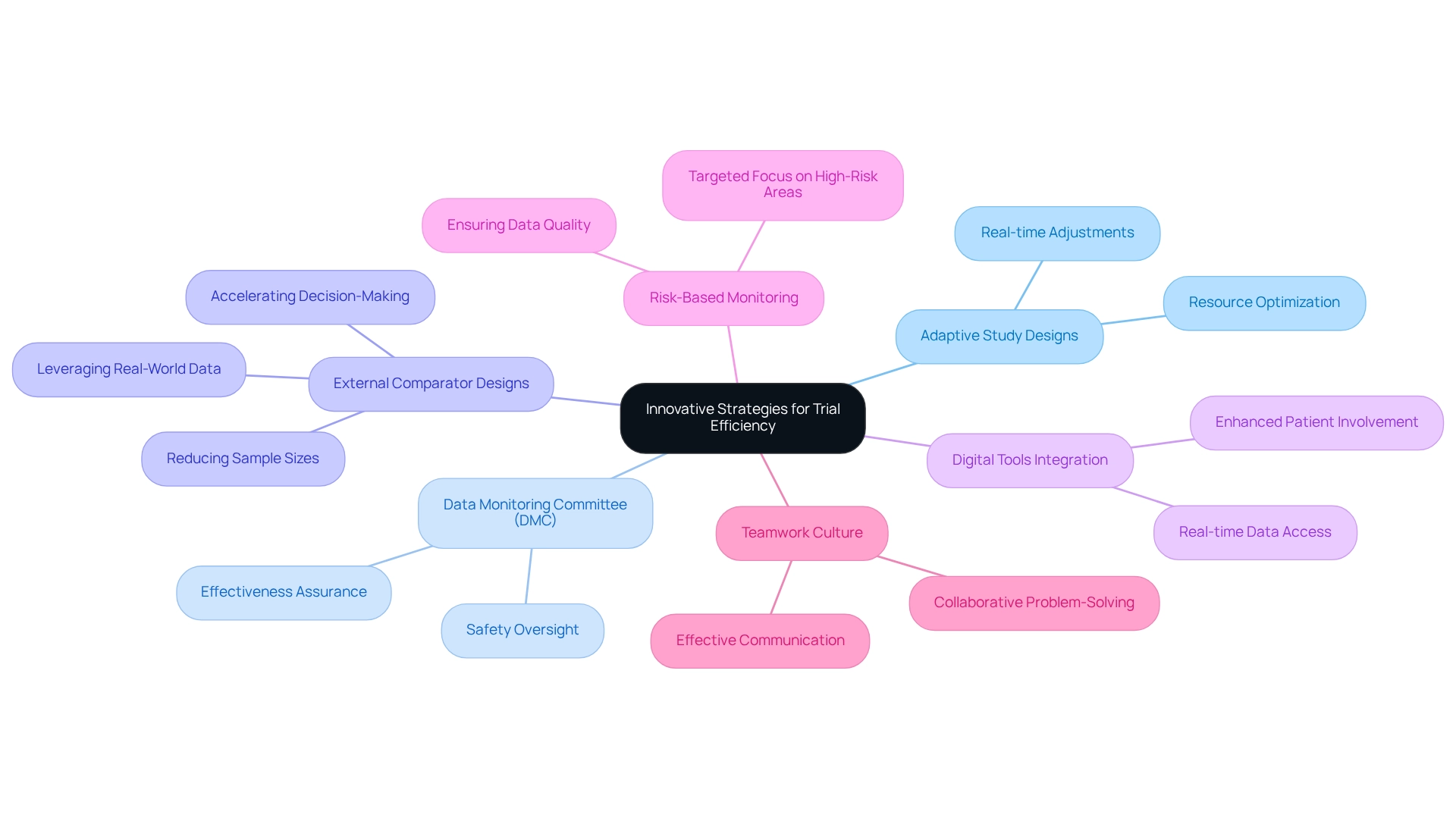
Innovative Strategies for Trial Efficiency
To enhance the effectiveness of medical device trial innovation in Brazil, stakeholders must embrace innovative strategies, particularly adaptive study designs that permit modifications based on interim results. This approach facilitates real-time adjustments and optimizes resource allocation, ultimately leading to more robust outcomes. The Data Monitoring Committee (DMC) is crucial in overseeing clinical studies, ensuring that subjects are not exposed to unsafe or ineffective treatments, thereby underscoring the significance of safety in adaptive study designs.
For instance, the application of external comparator designs leverages real-world data, potentially reducing sample sizes and accelerating informed decision-making, thus strengthening the overall study results. This design significantly boosts the efficiency of experiments by providing comparative context that substantiates the findings.
Integrating digital tools for data collection and patient monitoring can greatly streamline processes and reduce administrative burdens. These technologies enable real-time data access and enhance patient involvement, which is vital for maintaining study momentum. Additionally, implementing risk-based monitoring approaches allows for a targeted focus on high-risk areas, ensuring superior data quality and compliance with regulatory standards.
It is imperative to prepare clear patient information sheets that encompass all possible adaptations at the study's outset, offering practical guidance for stakeholders engaged in adaptive design studies.
Fostering a culture of teamwork among all participants—including sponsors, contract research organizations (CROs) like bioaccess®, and regulatory authorities—can lead to more effective communication and problem-solving throughout the study process. With over 20 years of experience in Medtech, bioaccess® is strategically positioned to navigate these complexities and drive successful outcomes. As Philip Pallmann highlighted, "All authors read and approved the final manuscript," emphasizing the importance of thorough review and collaboration in medical research.
By emphasizing these innovative approaches, Brazil can position itself as a leader in medical device trial innovation, ultimately propelling the advancement of health technologies and enhancing patient outcomes.
The Role of Collaboration in Trial Success
Cooperation among stakeholders is crucial for the success of medical device trial innovation in Brazil. Involving local healthcare providers, patient advocacy organizations, and regulatory bodies not only facilitates smoother study execution but also significantly enhances patient recruitment efforts. For instance, partnerships with academic institutions can unlock access to critical resources and expertise, fostering innovation and improving trial outcomes.
To effectively engage local healthcare providers, strategies such as tailored outreach programs and educational initiatives can be employed. These efforts should focus on:
- Showcasing the value of involvement in research
- Addressing any concerns regarding ethical considerations
- Emphasizing the potential advantages for both patients and the healthcare system
As one participant noted, "Setting research priorities should receive more attention. How do we allocate scarce resources fairly and in an equal manner? Treatments and medication become more and more expensive, and we can’t afford everything. It is important that research producing social value is executed in the most effective manner."
Moreover, regular communication and joint problem-solving sessions among stakeholders are vital. These interactions help to identify and address challenges as they arise, ensuring that all parties remain aligned and focused on common objectives. However, practical obstacles such as administrative hurdles and language barriers must also be considered to achieve effective collaboration.
Statistics underscore the importance of collaboration; for example, health researchers per million inhabitants vary significantly by income group, as reported by WHO in 2023. This disparity highlights the need for collaborative efforts to enhance research capacity in lower-income regions. Success stories from Brazil illustrate how effective collaborations between contract research organizations (CROs) such as bioaccess™ and healthcare providers have contributed to medical device trial innovation, resulting in more successful evaluations that can benefit diverse populations.
Significantly, GlobalCare Clinical Studies' partnership with bioaccess™ has led to an extraordinary decrease in participant recruitment time by over 50% and an increase in participant retention rates of over 95%, highlighting the concrete advantages of collaboration.
Furthermore, bioaccess™ provides extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These services are essential for ensuring that research studies are carried out efficiently and effectively, ultimately aiding in the enhancement of local economies through job creation and improved healthcare results.
A case study titled 'Enhancing Community Awareness in Clinical Research' highlights the necessity for greater community involvement concerning the ethical dimensions of research, which is vital for participant recruitment and overall success.
In conclusion, the incorporation of cooperative approaches among stakeholders is not only advantageous but essential for progressing healthcare evaluations in Brazil, especially regarding medical device trial innovation, ensuring that research generates significant social value and improves public health outcomes.
Effective Patient Recruitment and Retention Strategies
Efficient patient recruitment and retention approaches are vital for the success of medical device trial innovation in Brazil. Community outreach initiatives are instrumental in enhancing awareness and participation in research studies, particularly given that 24.1% of respondents identified a lack of research awareness as a significant barrier. By leveraging Brazil's extensive public healthcare network, researchers can engage diverse communities and foster greater involvement.
bioaccess®, a leading Contract Research Organization, is at the forefront of supporting healthcare technology studies in Latin America. Its specialized expertise and tailored approach are crucial for expediting the advancement of medical devices for companies in the Medtech industry. Customizing recruitment messages to resonate with local communities while addressing cultural sensitivities can markedly boost participation rates.
Successful case studies in Brazil illustrate that localized outreach efforts—such as informational sessions and collaborations with community leaders—can effectively enhance awareness and encourage enrollment. Insights from the Horizon Databook further underscore the importance of these strategies, providing valuable data on trends and projections within Brazil's medical studies market. Moreover, bioaccess's partnership with Caribbean Health Group to establish Barranquilla as a premier hub for medical studies in Latin America, supported by Colombia's Minister of Health, exemplifies the potential for strategic collaborations to elevate research capabilities in the region.
This partnership is designed to streamline clinical research processes and improve patient access to innovative therapies, showcasing bioaccess's commitment to advancing healthcare in Latin America. Once participants are enrolled, it is crucial to maintain their interest for retention. Regular communication—comprising updates on progress and personalized assistance—fosters a sense of engagement and commitment.
Additionally, offering incentives, such as travel reimbursements or access to new treatments, can encourage participants to stay engaged throughout the study duration. By implementing these effective patient recruitment strategies, medical device trial innovation in Brazil can achieve enhanced enrollment and retention rates, ultimately leading to more favorable outcomes. The authors express gratitude to Hoffmann-La Roche Ltd, Switzerland, for their support of this research.
Leveraging Technology for Clinical Trial Innovation
Utilizing technology is essential for fostering innovation in medical device trials in Brazil, particularly within the context of the country's evolving landscape. Digital tools, such as electronic data capture (EDC) systems, remote monitoring devices, and telemedicine platforms, are revolutionizing data collection methods and elevating patient engagement. The anticipated integration of EDC systems in 2025 is poised to significantly improve efficiency in studies, facilitating more accurate and timely data management.
Artificial intelligence (AI) plays a pivotal role in this transformation. By leveraging AI for data analysis, stakeholders can enhance their decision-making capabilities and reveal trends that may not be readily apparent through traditional approaches. Furthermore, mobile applications designed for patient interaction provide immediate feedback, ensuring that participants remain engaged and informed throughout the study process.
The impact of these technological advancements extends beyond operational efficiency; they also contribute to cost savings and improved research quality. As sponsors increasingly demand greater ownership and transparency of their data, the trend towards insourcing data management processes is becoming increasingly evident. This shift not only empowers sponsors but also enhances the overall quality and operational efficiency of research studies, ultimately benefiting patients and advancing the healthcare sector in Latin America through medical device trial innovation in Brazil.
In this context, bioaccess® distinguishes itself with its comprehensive research management services, encompassing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). As Max Baumann emphasized, there is a sustained focus on optimizing the development journeys of assets to achieve not only approval-enabling endpoints but also to secure commercial success. Additionally, the case study titled 'Increased Data Ownership and Transparency for Sponsors' exemplifies how sponsors are pursuing greater control over their data, aligning with the ongoing discussion regarding the insourcing of data management processes.
Looking ahead to 2025, study sponsors are expected to adopt a core outcome set across various study designs, further standardizing clinical research practices and enhancing the impact of Medtech clinical studies on local economies through job creation, economic growth, and healthcare improvement.
Key Takeaways and Best Practices for Future Trials
Successful medical device trial innovation in Brazil requires a comprehensive approach that encompasses a thorough understanding of the local environment, careful navigation of regulatory compliance, and the application of innovative strategies. Key takeaways for achieving success in this dynamic environment include:
- Collaboration Among Stakeholders: Engaging with local partners, regulatory bodies, and healthcare professionals is essential for fostering trust and ensuring that studies are culturally sensitive and ethically sound. This collaboration enhances the significance of research results and enables smoother testing processes.
- Effective Patient Recruitment and Retention: Developing targeted recruitment strategies that resonate with the local population is crucial. This involves employing community outreach and education to inform prospective participants about the advantages of clinical studies, thereby enhancing enrollment rates and retention. Importantly, the informed consent form (ICF) must be provided in a language the participant comprehends, ensuring clarity and understanding.
- Utilizing Technology: Incorporating advanced technologies can significantly improve study efficiency. Employing electronic data capture systems, telemedicine, and mobile health applications simplifies procedures and enhances participant involvement.
In 2025, optimal methods for medical device studies in Brazil also emphasize the significance of adhering to ethical guidelines, particularly when collaborating with indigenous communities. Research involving these populations must respect their unique cultural contexts and ensure that the benefits align with their needs, fostering trust and collaboration. By adhering to specific ethical standards, researchers can guarantee that their work is both moral and advantageous to indigenous communities.
Moreover, the recent classification of multiple foreign regulatory bodies as Equivalent Foreign Regulatory Authorities (AREE) by ANVISA has streamlined the assessment process for medical device trial innovation in Brazil. This designation simplifies the regulatory environment for stakeholders, facilitating navigation for companies through the process and ultimately resulting in quicker access to innovative solutions that enhance patient outcomes. As Juan Cuya, MD, Clinical Trial Associate at bioaccess®, emphasizes, aligning practices with INVIMA's guidelines is crucial for ensuring successful research outcomes.
By adopting these best practices and leveraging the evolving regulatory environment, stakeholders can significantly enhance their chances of success through medical device trial innovation in Brazil. Additionally, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). bioaccess® is committed to ensuring information security and client trust, with established grievance and data protection procedures to address any concerns regarding data handling, thereby reinforcing transparency and compliance in all clinical trial activities.
Conclusion
Brazil's medical device market presents a dynamic and promising landscape, marked by robust growth driven by government initiatives, increasing middle-class demand, and shifting societal attitudes toward healthcare. Navigating this intricate environment requires a comprehensive understanding of local dynamics, regulatory challenges, and cultural nuances. Stakeholders must prioritize collaboration with local healthcare providers and regulatory bodies to optimize trial execution and patient recruitment, ultimately leading to more effective and relevant clinical research.
The complexities of conducting medical device trials are intensified by stringent regulatory requirements, logistical hurdles, and the necessity for ethical compliance. Nevertheless, strategic planning, innovative trial designs, and the integration of technology can alleviate these challenges and streamline processes. By embracing adaptive trial designs and leveraging digital tools, stakeholders can enhance efficiency, improve patient engagement, and ensure data integrity.
Looking forward, the synergy of collaborative efforts and technological innovations will be pivotal in advancing medical device trials in Brazil. By emphasizing effective patient recruitment strategies, adherence to ethical standards, and continuous engagement with local communities, stakeholders can ensure that clinical research yields meaningful social value and contributes to enhanced healthcare outcomes. Organizations like bioaccess® are committed to navigating these complexities and facilitating successful trials, positioning Brazil as an emerging leader in the medical device sector, ultimately benefiting both the economy and public health.
Frequently Asked Questions
What is the significance of Brazil's healthcare device market in Latin America?
Brazil's healthcare device market is one of the largest in Latin America, characterized by a diverse range of products and driven by innovations in medical device trials.
How does Brazil's healthcare system influence the adoption of healthcare technologies?
Brazil's healthcare system, which includes both public and private sectors, plays a crucial role in shaping the adoption of healthcare technologies through government initiatives aimed at improving healthcare quality and accessibility.
What recent trends are affecting the demand for healthcare tools in Brazil?
The growing middle class in Brazil is increasing demand for advanced healthcare tools, particularly in the aesthetic procedures sector, which is seeing rising interest among men.
What challenges does Brazil's demographic landscape present for medical device trial innovation?
Brazil's diverse population has varying health needs across different regions, creating both opportunities and challenges for medical device trial innovation that stakeholders must navigate.
What services does bioaccess® provide for medical device trials in Brazil?
Bioaccess® offers comprehensive trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
What are the regulatory challenges faced in medical device trials in Brazil?
The Brazilian Health Regulatory Agency (ANVISA) imposes strict requirements that can prolong approval processes, complicating the initiation of medical device trials.
How do ethical approvals and compliance impact medical device trials in Brazil?
Local ethical approvals and adherence to Good Clinical Practice (GCP) introduce additional complexities, requiring comprehensive documentation and compliance with various regulatory standards.
What logistical hurdles are encountered in recruiting patients for medical device trials in Brazil?
Researchers face challenges in patient recruitment due to cultural differences and varying levels of awareness regarding research studies, which can hinder the success of trials.
Why is the registration of research studies important in Brazil?
The registration of research studies is seen as a scientific, ethical, and moral obligation, particularly in the context of medical device trials, emphasizing integrity and respect for participants.
What strategies can help navigate the challenges of medical device trial innovation in Brazil?
Proactive planning, engaging local experts early in the process, and understanding the regulatory landscape are essential strategies for overcoming challenges in medical device trial innovation.
What recent initiatives highlight the importance of inclusive approaches in medical device trials?
Initiatives focusing on gender-affirming therapies, such as studies on hormone therapy interactions with HIV medications in trans women, underscore the need for inclusive approaches that consider diverse participant demographics.




