Overview
Latin America stands out as a prime location for clinical research, driven by its demographic diversity, progressive regulatory frameworks, and an expanding research infrastructure. These elements collectively enhance the feasibility and effectiveness of clinical trials. The region's unique patient populations and economic advantages are significant, as they facilitate successful collaborations that streamline research processes.
Consequently, Latin America emerges as an appealing destination for pharmaceutical companies aiming to conduct diverse and impactful studies.
How can your organization leverage these opportunities to overcome challenges in clinical research?
Introduction
Latin America is rapidly emerging as a key player in the global clinical research landscape, driven by its diverse demographics, evolving regulatory frameworks, and a commitment to innovation.
With a significant treatment-naive population and a high prevalence of diseases such as oncology and diabetes, the region offers a unique environment for conducting clinical trials that yield valuable insights into treatment efficacy and safety.
As global pharmaceutical companies seek to expand their clinical trial portfolios, Latin America’s rich cultural and genetic diversity enhances the generalizability of research findings, making it an attractive option for advancing medical research.
Collaborations among local organizations and government support further position the region as a hub for cutting-edge clinical studies, paving the way for impactful medical advancements.
The Rising Appeal of Latin America for Clinical Research
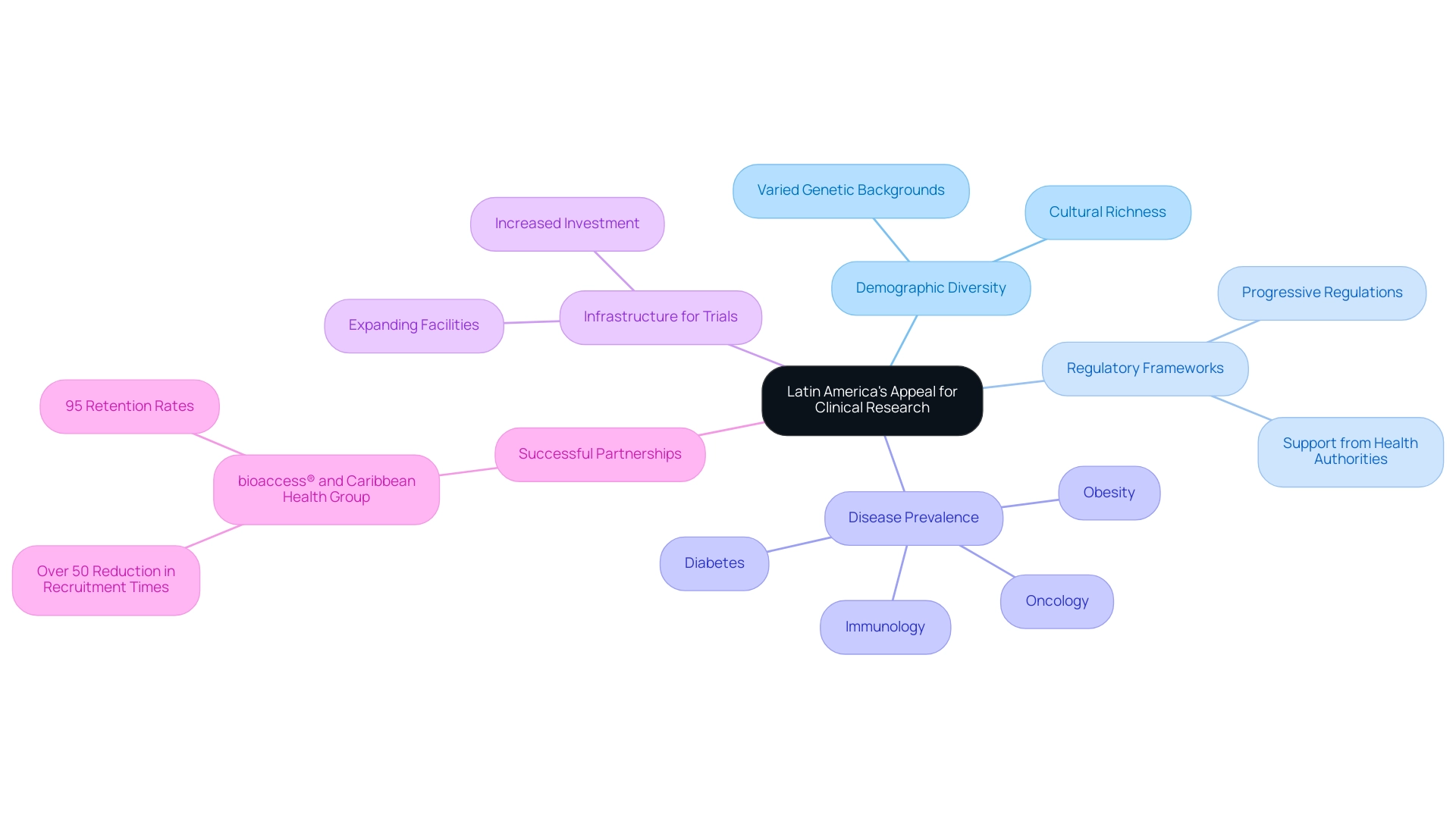
The factors contributing to Latin America's appeal for clinical research are its demographic diversity, progressive regulatory frameworks, and an expanding infrastructure for trials, positioning the region as a prime destination for research studies. As of February 2025, the number of registered research studies in Argentina underscores the area's growing significance in this field. The region features a substantial treatment-naive population, essential for obtaining unbiased data, alongside a high prevalence of diseases such as oncology, immunology, diabetes, and obesity.
These elements create an ideal environment for conducting research studies that can yield significant insights into treatment efficacy and safety. The cultural richness and varied genetic backgrounds of Latin American populations further enhance the generalizability of findings. This diversity allows for a more thorough comprehension of how various demographics respond to medical interventions, making the area particularly attractive to international pharmaceutical firms and organizations aiming to expand their study portfolios.
Successful partnerships, such as those between bioaccess® and Caribbean Health Group, demonstrate the region's capacity to provide valuable insights to the global medical community. This collaboration aspires to establish Barranquilla as a premier destination for medical trials in Latin America, supported by Colombia's Minister of Health, who has publicly endorsed efforts to enhance health project initiatives in the region. These collaborations have yielded remarkable outcomes, including over a 50% reduction in recruitment times and retention rates soaring to 95%.
As Gotuzzo from Universidad Peruana Cayetano Heredia observes, "These challenges require a strategic approach to recruitment," underscoring the importance of effective strategies in overcoming barriers in the scientific investigation landscape. As the demand for innovative medical studies continues to grow, particularly in fields like oncology and diabetes, researchers are examining why Latin America is emerging as a key participant in the global trial arena. The region's distinctive demographic traits and evolving regulatory landscape highlight its attractiveness for promoting medical studies and facilitating the rapid advancement of new therapies. With bioaccess® leading the way in comprehensive trial management services—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—the potential for impactful medical advancements is substantial.
Advancements in Clinical Research Infrastructure
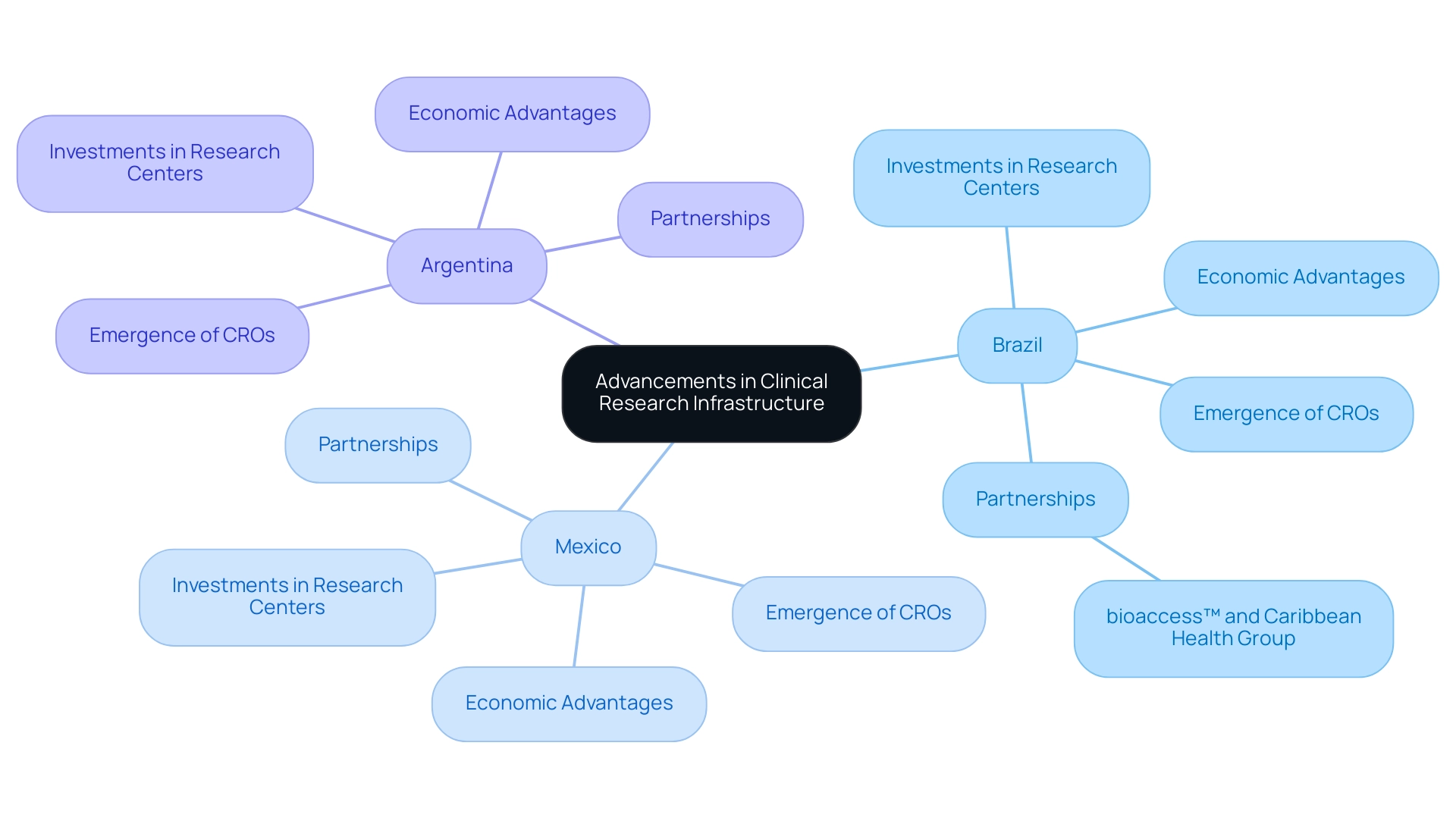
Recent years have witnessed remarkable progress in the medical study framework throughout Latin America, particularly in Brazil, Mexico, and Argentina. These nations have made substantial investments to enhance their investigative capabilities, resulting in the establishment of specialized centers and state-of-the-art laboratory facilities. Such advancements are crucial, as they align with global norms for medical studies, significantly enhancing the trustworthiness of investigations conducted in the region.
The unique combination of economic advantages, diverse populations, and a commitment to health equity elucidates why Latin America is increasingly viewed as a strategic option for pharmaceutical companies seeking clinical research opportunities. The emergence of local Contract Research Organizations (CROs) in Brazil, Mexico, and Argentina has streamlined operations and optimized resource management, further illustrating the region's appeal for clinical research. For instance, Brazil's commitment to promoting innovation is evident in its funding of medical study centers, which are well-equipped to manage intricate investigations and cater to various patient groups.
This investment is bolstered by Colombia's generous R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, which significantly enhances the region's attractiveness.
Expert opinions underscore the growing capabilities of these nations in conducting health studies. As Dr. Sergio Alvarado, a Clinical Trial Manager focused on innovative medical research, observes, "The adaptability and continuous learning within the region's workforce are crucial for meeting the evolving demands of research." This adaptability is evidenced by the increasing number of research facilities being established, all aimed at meeting the stringent standards anticipated by global pharmaceutical firms.
Moreover, partnerships such as the one between bioaccess™ and the Caribbean Health Group are striving to position Barranquilla as a prominent hub for medical studies in Latin America, supported by Colombia's Minister of Health. This collaboration is poised to enhance the region's medical research landscape, creating job opportunities and fostering economic growth. Additionally, bioaccess™ offers extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
These capabilities are vital for ensuring that research studies are conducted effectively and in accordance with regulatory standards.
Case studies, such as the GlobalCare Clinical Trials collaboration with bioaccess™, exemplify the success of local initiatives, achieving over a 50% reduction in recruitment time and 95% retention rates in research studies. As the region continues to embrace innovation and patient-focused practices, it is poised to become a prominent center for medical studies, illustrating why Latin America attracts clinical research by providing distinct advantages for pharmaceutical firms aiming to conduct studies in a diverse and economically favorable environment.
Prioritizing Patient-Centric Approaches in Trials
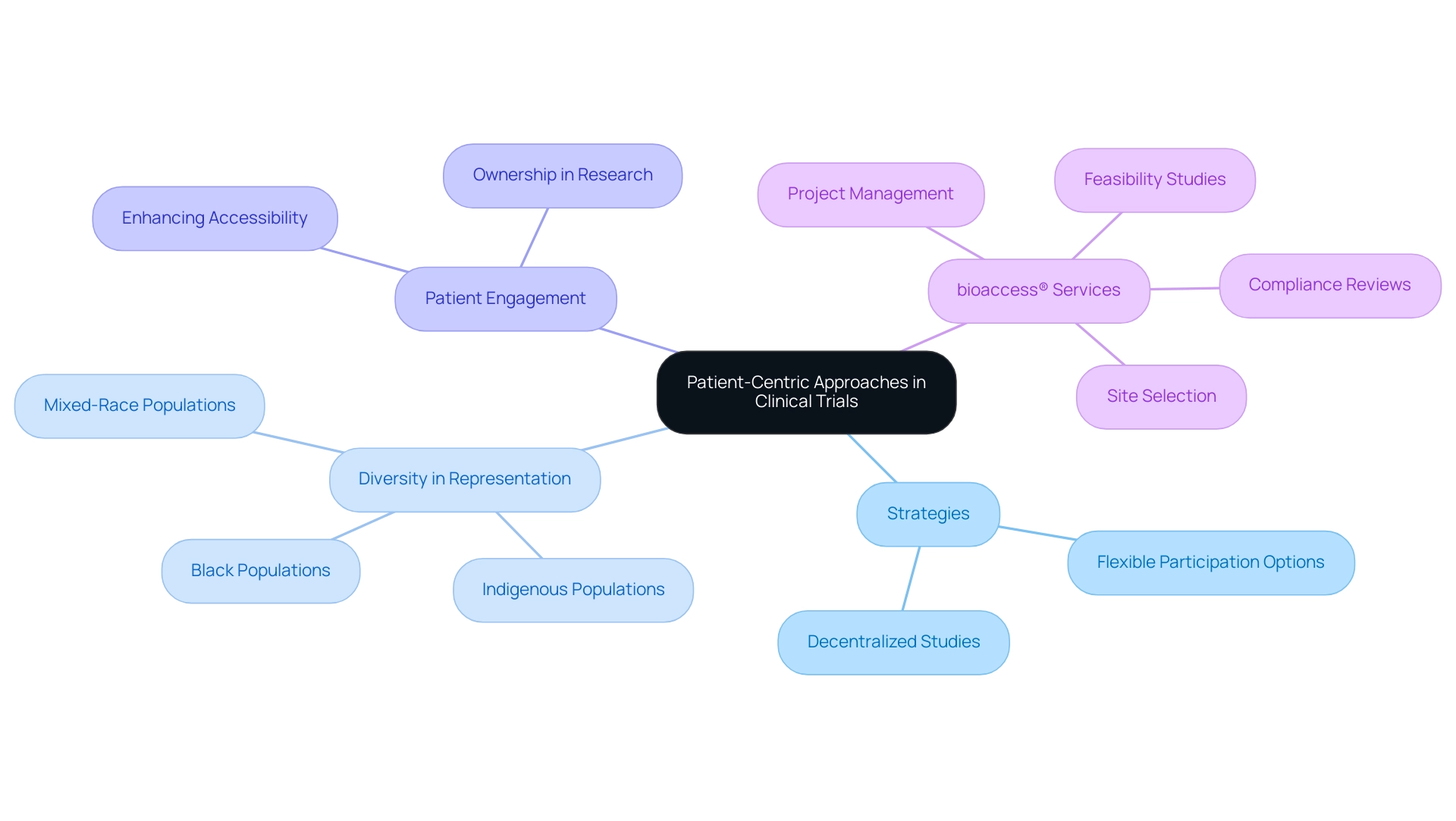
In recent years, Latin America has undergone a significant transformation towards patient-centric methods in clinical studies, a shift that aligns with bioaccess®'s commitment to facilitating innovative medical device research in the region. This transformation underscores the critical importance of actively involving patients in the research process, ensuring that their needs and preferences are at the forefront. By implementing strategies such as decentralized studies and offering flexible participation options, researchers can significantly enhance patient engagement and retention, ultimately driving global health improvement through international collaboration and innovation in Medtech.
Decentralized studies, which enable remote participation and data collection, have proven particularly effective in enhancing accessibility for diverse patient populations. This method not only enhances the overall experience but also cultivates a sense of ownership among participants, resulting in higher quality data and more dependable outcomes. For instance, Brazil's multiethnic population presents a distinctive opportunity for medical studies; however, current participation rates are disproportionately biased towards white Caucasian individuals.
Efforts are underway to increase representation from Indigenous, mixed-race, and Black populations, which is crucial for ensuring that findings reflect the broader population's needs and characteristics. As Karla Espirito Santo of Einstein observed, "The concept is to enhance the variety of contributors in research studies, and not just among the limited group of patients already visiting research centers."
Furthermore, addressing obstacles to informed consent is vital for improving patient safety and the standard of research in the area. Effectively tackling these barriers can enhance patient safety and the overall quality of research studies. By prioritizing patient-centric methodologies, researchers can create a more inclusive environment that benefits participants and sponsors alike, ultimately accelerating the development of effective treatments tailored to diverse genetic and socioeconomic backgrounds.
bioaccess® plays a crucial role in this landscape by providing extensive research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These capabilities guarantee that research studies are conducted effectively and in accordance with local regulations, which is essential for achieving successful results.
As the landscape of medical studies continues to evolve, the emphasis on patient involvement and decentralized studies will be critical in understanding why Latin America is an attractive destination for clinical research. This focus is further bolstered by partnerships such as that of bioaccess™ and Caribbean Health Group, which aim to establish Barranquilla as a prominent site for medical evaluations.
Cost-Effectiveness and Economic Advantages
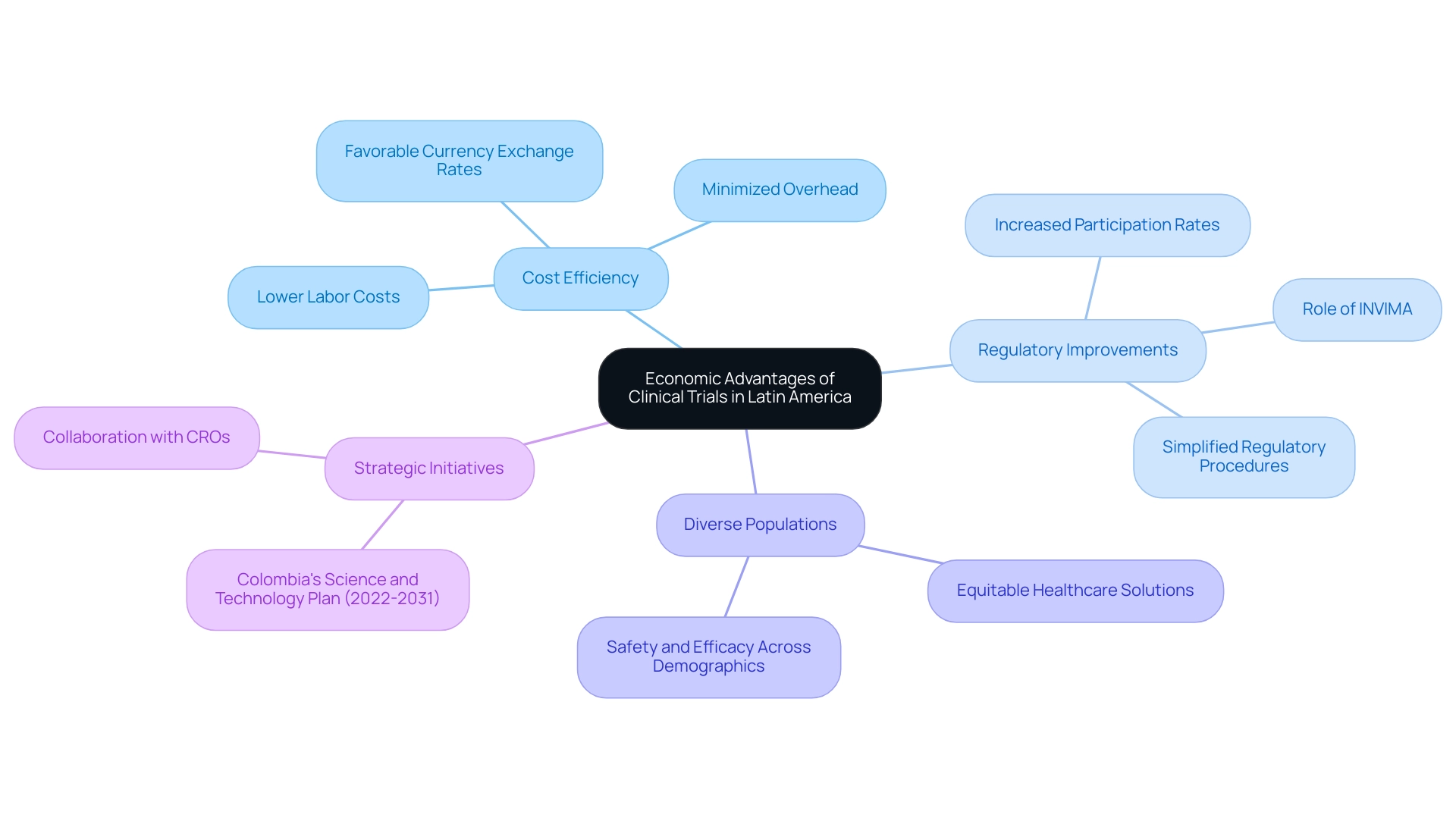
The substantial economic benefits of conducting medical trials in Latin America clearly illustrate why the region attracts clinical research, making it an appealing choice for Medtech firms. Notably, the cost-efficiency of investigative activities stands out, with studies in Latin America generally being significantly cheaper than those in North America and Europe. This reduction in expenses can be attributed to lower labor costs, minimized overhead, and favorable currency exchange rates.
Such economic advantages empower sponsors to enhance their budgets, allowing for the potential of larger studies or the distribution of funds to further initiatives.
Moreover, the area's commitment to improving the medical study environment is evident through simplified regulatory procedures and increased participation rates. These factors not only facilitate faster patient recruitment but also contribute to a more efficient study timeline. The collaboration of legislative backing and an emphasis on diversity in medical studies further positions Latin American contract organizations (CROs) as essential allies for Medtech companies.
By embracing diverse populations, these studies can lead to more equitable healthcare solutions, ensuring that new treatments are safe and effective across various demographic groups.
As highlighted by Julio G. Martinez-Clark, CEO of bioaccess®, "Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy." This strategic emphasis enhances the region's attractiveness for conducting medical research, underscoring why Latin America is a preferred option for Medtech firms seeking to advance their innovations rapidly and affordably. Furthermore, understanding the intricacies of the regulatory landscape, particularly the role of INVIMA as a Level 4 health authority overseeing medical device regulations, is crucial for successful research in the area.
With over 20 years of experience in the Medtech sector, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. Additionally, bioaccess® has partnered with Avantec Vascular for a first-in-human study in Latin America, showcasing its capability to assist companies in navigating these challenges and capitalizing on the benefits of conducting research in the region.
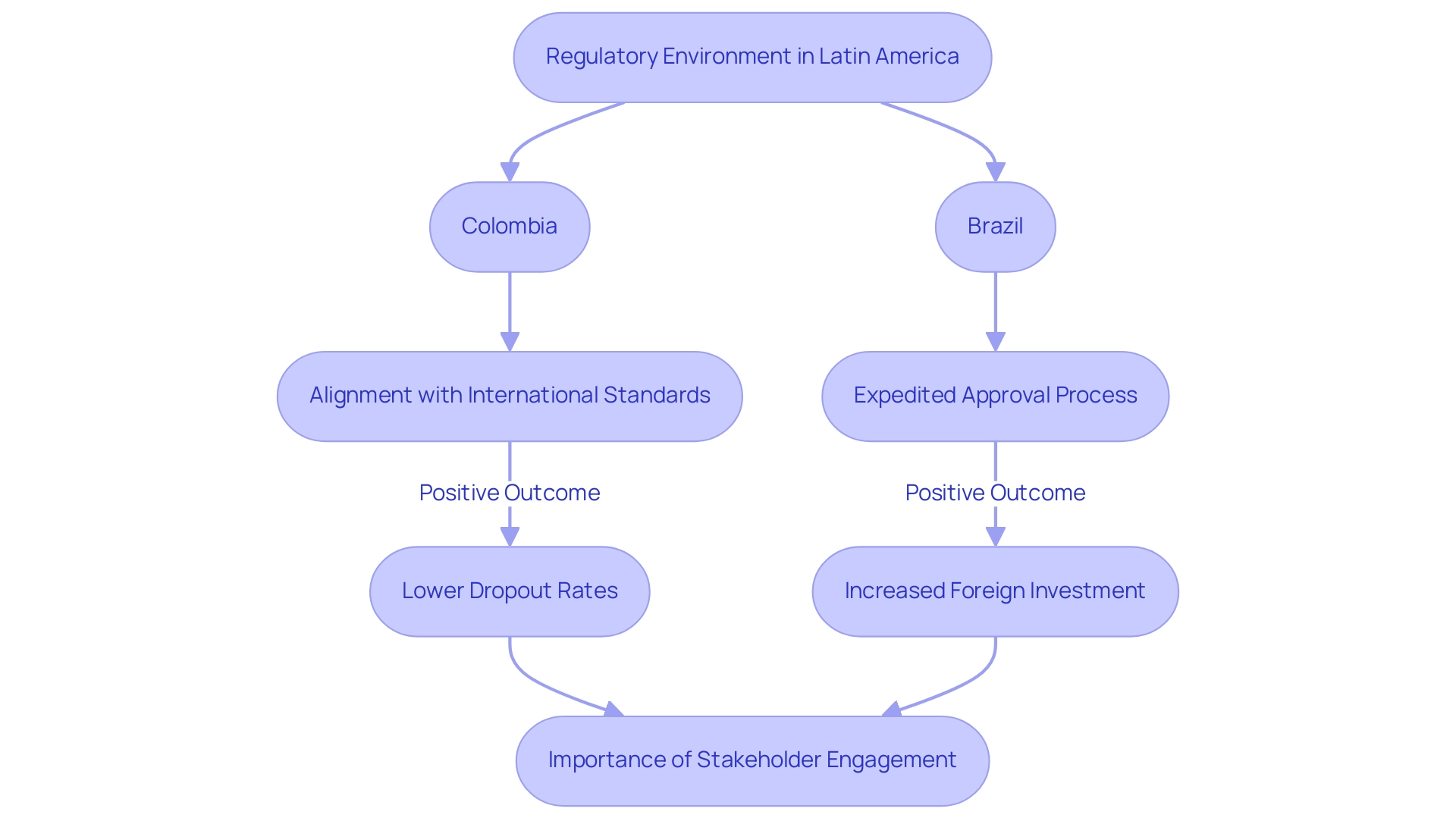
Navigating the Evolving Regulatory Landscape
The regulatory environment for medical research in Latin America is undergoing a significant transformation, with countries like Colombia actively implementing reforms aimed at simplifying processes and enhancing compliance. Regulatory bodies, including INVIMA (Colombia's National Food and Drug Surveillance Institute), are increasingly aligning with international standards, which not only improves the quality of studies but also encourages greater collaboration with global sponsors. Notably, Colombia has received a Level 4 health authority classification from PAHO/WHO, highlighting its commitment to upholding high regulatory standards.
In Brazil, recent legislative changes have been pivotal in expediting the research approval process, effectively reducing bureaucratic hurdles and enhancing predictability for both researchers and sponsors. These evolving regulations are essential for attracting foreign investment, ensuring that research in the region adheres to the highest quality and safety standards.
Furthermore, the impact of these regulatory shifts is underscored by a compelling statistic: dropout rates in Latin America are approximately one-third lower than those in the U.S. and EU, illustrating the region's potential for successful outcomes. As Julio G. Martinez-Clark, CEO of bioaccess®, notes, "Colombia has acknowledged the advantages of research regulation in Latin America and has created an ambitious science, technology, and innovation strategy for 2022–2031 to transition into a knowledge-based economy."
The importance of ongoing interaction with stakeholders cannot be overstated, as it is crucial for addressing challenges and facilitating smoother processes throughout research studies. A case study titled "Building Partnerships: Collaborating with Local Stakeholders" exemplifies this, emphasizing that understanding patient populations and refining recruitment strategies are vital for success. This partnership has been instrumental in demonstrating the benefits of study regulations in Latin America, showcasing how local engagement can enhance outcomes.
In addition to these insights, effective strategies for pioneering medical studies in Latin America involve efficient methodologies for conducting successful experiments in this dynamic landscape. As the regulatory environment continues to evolve, it is imperative for healthcare professionals to remain informed about Brazil's legislative changes and their broader implications for medical studies in the region. The current reforms not only promise to improve the efficiency of the approval process but also highlight the reasons why Latin America is becoming a competitive player in the global clinical research arena, with bioaccess® leading the way in supporting medical device evaluations.
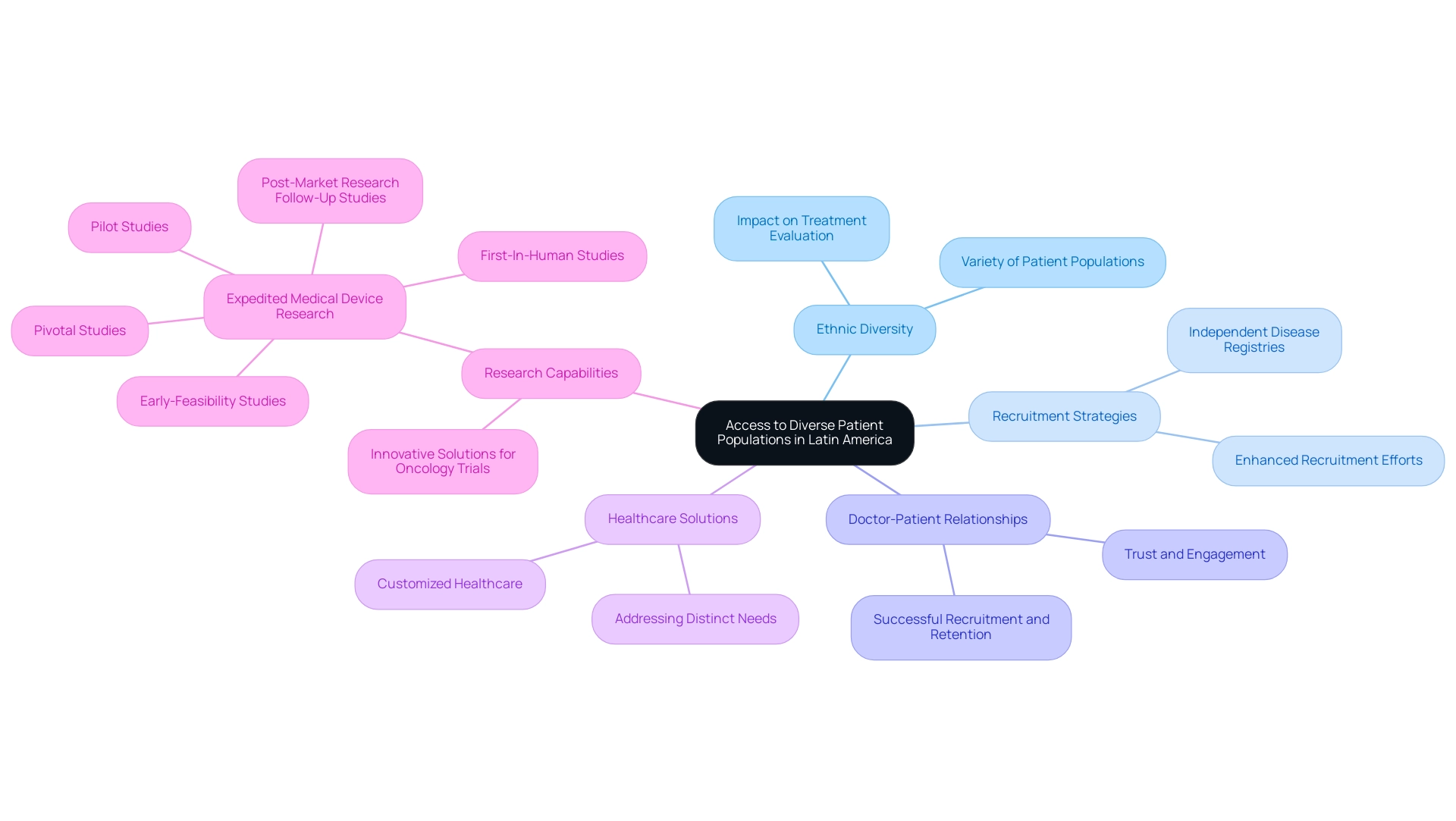
Access to Diverse Patient Populations
Latin America boasts a rich diversity of patient populations, encompassing a wide array of ethnic backgrounds and health conditions. This ethnic variety is a crucial asset for clinical trials, underscoring why Latin America attracts clinical research; it enables researchers to evaluate treatment effects across different demographic groups. Access to such diverse patient populations enhances recruitment efforts and significantly improves the generalizability of research findings.
In 2025, the focus on understanding ethnic backgrounds in health statistics illustrates that customized healthcare solutions are essential for addressing the distinct needs of patients in the region.
Moreover, the strong doctor-patient relationships prevalent in Latin America are pivotal in attracting clinical research, fostering an environment of trust and engagement vital for successful recruitment and retention in research studies. A case study titled "Understanding Patient Diversity in Latin America" emphasizes the importance of this diversity, demonstrating that recognizing and addressing the varied needs of patients can lead to improved healthcare outcomes. This case study serves as an authoritative guide for healthcare professionals, highlighting that understanding patient diversity is essential for fostering tailored healthcare solutions that effectively meet the varied needs of patients in the region.
In partnership with Caribbean Health Group, bioaccess™ is actively working to establish Barranquilla as a premier location for medical studies in Latin America, a decision supported by Colombia's Minister of Health. This initiative aims to enhance the experimental landscape, making it more appealing for global projects. As efforts to improve diversity in research enrollment continue, strategies such as recruiting from independent disease registries are being implemented to yield comprehensive data and enhance study outcomes.
These ongoing initiatives underscore why Latin America attracts clinical research, emphasizing the significance of diversity in medical studies to deepen our understanding of how various populations respond to treatments.
The advantages of ethnic variety in research extend beyond recruitment; they also provide insights into why Latin America attracts clinical research by enhancing our understanding of how different populations respond to treatments. This insight is particularly valuable in oncology research, where the unique challenges presented by diverse patient groups can be effectively addressed through innovative solutions. The diverse array of cultural backgrounds in Latin America contributes to substantial and relevant outcomes in scientific studies, reinforcing why Latin America attracts clinical research and ultimately advancing the field of medical technology.
Additionally, bioaccess® offers expedited medical device research study services, focusing on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Research Follow-Up Studies (PMCF), further enhancing the region's capabilities in conducting high-quality research.
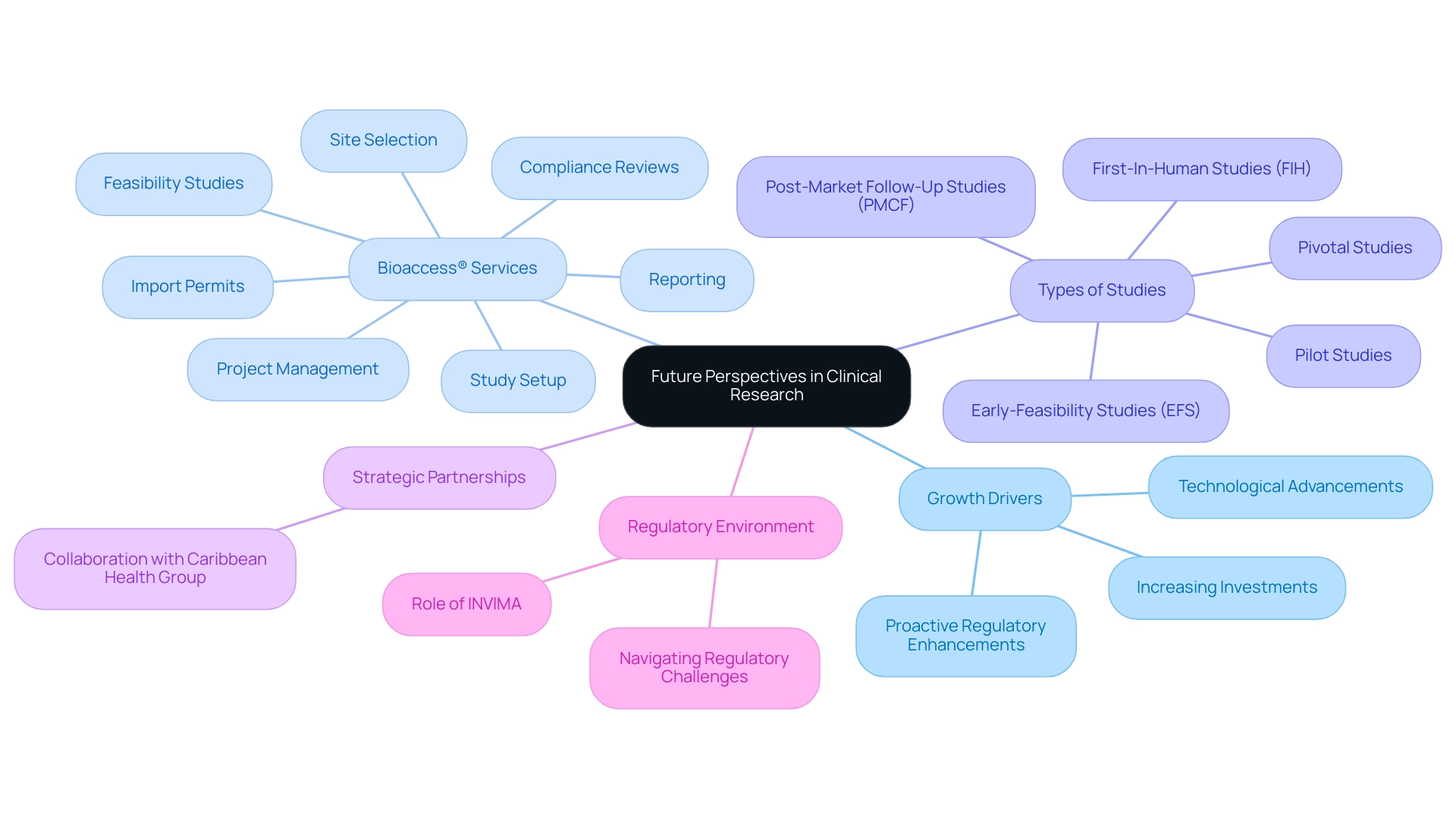
Future Perspectives: Growth and Innovation in Clinical Research
The prospects for health studies in Latin America are exceptionally promising, underscoring the region's appeal for clinical research. Predictions indicate strong growth, driven by increasing investments, technological advancements, and a proactive approach to regulatory enhancements. As international stakeholders increasingly recognize the strategic advantages of conducting assessments in this region, the demand for medical study services is anticipated to rise. Notably, developments such as decentralized studies and the integration of digital health technologies are transforming the investigative landscape, enhancing accessibility and efficiency in study implementation.
Moreover, extensive management services provided by bioaccess®, including:
- feasibility studies
- site selection
- compliance reviews
- study setup
- import permits
- project management
- reporting
are essential for navigating the complexities of medical studies. Bioaccess® specializes in various medical device research studies, such as:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Strategic partnerships between local contract organizations (CROs) and international entities, exemplified by the collaboration with Caribbean Health Group, are expected to significantly enhance study registration rates and streamline patient recruitment processes, reinforcing Latin America's position as a pivotal player in the global trial sector.
This collaboration aims to establish Barranquilla as a leading location for medical studies, supported by Colombia's Minister of Health, emphasizing the region's commitment to advancing its research capabilities.
The anticipated highest compound annual growth rate (CAGR) in Argentina within the Latin American research market from 2024 to 2030 further illustrates the region's potential as a hub for innovative medical exploration. The role of INVIMA, Colombia's National Food and Drug Surveillance Institute, as a Level 4 health authority recognized by PAHO/WHO, is crucial for ensuring regulatory compliance and oversight for medical devices, contributing to Latin America's appeal for clinical research. As highlighted in the case study "Navigating the Medtech Regulatory Landscape in Latin America," understanding the intricacies and challenges within the regulatory environment is essential for stakeholders engaged in trials.
Additionally, it is noteworthy that the Asia Pacific region is projected to reach USD 25,992.8 million by 2030, providing a comparative backdrop for the growth of medical studies in Latin America. As Roshan Deshmukh aptly stated, "The future of medical exploration in Latin America is filled with unique opportunities that can address both local and global health needs," exemplifying why Latin America is poised to attract clinical research in 2025 and beyond.
Conclusion
Latin America is emerging as a pivotal hub for clinical research, driven by its diverse patient populations, evolving regulatory frameworks, and a robust commitment to innovation. The region's unique demographic characteristics, including a significant treatment-naive population and a high prevalence of diseases such as oncology and diabetes, foster an ideal environment for conducting clinical trials that yield valuable insights into treatment efficacy and safety.
The advancements in clinical research infrastructure, particularly in countries like Brazil, Mexico, and Argentina, further enhance the region's appeal. Investments in specialized research centers and local Contract Research Organizations (CROs) have streamlined operations, making it increasingly attractive for global pharmaceutical companies seeking cost-effective solutions without compromising quality. Moreover, the emphasis on patient-centric approaches, including decentralized trials, signifies a transformative shift towards prioritizing patient needs, ultimately improving recruitment and retention rates.
As Latin America continues to navigate an evolving regulatory landscape, its commitment to aligning with international standards promises to enhance the credibility of research conducted in the region. The collaborative efforts between local organizations and government support are paving the way for significant medical advancements, ensuring that Latin America remains a competitive player in the global clinical research arena.
In summary, the combination of economic advantages, diverse demographics, and a robust regulatory framework positions Latin America as a strategic choice for innovative clinical research. The future appears promising, with increasing investments and technological advancements set to further solidify the region's role as a critical player in advancing global health solutions. As stakeholders continue to embrace these opportunities, the potential for impactful medical breakthroughs in Latin America is substantial.
Frequently Asked Questions
What factors contribute to Latin America's appeal for clinical research?
Latin America's appeal for clinical research is driven by its demographic diversity, progressive regulatory frameworks, and expanding infrastructure for trials, making it an ideal destination for research studies.
Why is the treatment-naive population important in clinical research?
The treatment-naive population is essential for obtaining unbiased data, which is crucial for assessing treatment efficacy and safety in clinical research.
What are some prevalent diseases in Latin America that attract clinical research?
The region has a high prevalence of diseases such as oncology, immunology, diabetes, and obesity, which are significant areas of focus for clinical studies.
How does demographic diversity enhance clinical research in Latin America?
The cultural richness and varied genetic backgrounds of Latin American populations allow for a more comprehensive understanding of how different demographics respond to medical interventions, enhancing the generalizability of research findings.
What successful partnerships have been established in Latin America for clinical research?
Partnerships, such as those between bioaccess® and Caribbean Health Group, aim to establish Barranquilla as a premier destination for medical trials, showcasing the region's ability to provide valuable insights to the global medical community.
What impact have collaborations had on recruitment and retention in clinical studies?
Collaborations have led to significant outcomes, including over a 50% reduction in recruitment times and retention rates reaching 95%.
What recent advancements have been made in the medical study framework throughout Latin America?
Countries like Brazil, Mexico, and Argentina have made substantial investments to enhance their investigative capabilities, establishing specialized centers and state-of-the-art laboratory facilities that align with global norms.
How do local Contract Research Organizations (CROs) affect clinical research in the region?
The emergence of local CROs has streamlined operations and optimized resource management, enhancing the region's attractiveness for clinical research.
What financial incentives does Colombia offer to boost clinical research?
Colombia offers generous R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, which significantly enhance the region's appeal for pharmaceutical companies.
What services does bioaccess® provide to support clinical research?
Bioaccess® offers comprehensive trial management services, including feasibility studies, site selection, compliance reviews, project management, and reporting, ensuring effective and regulatory-compliant research studies.




